Abstract
Purpose
Exposure to ionizing radiation, especially during childhood, is a well-established risk factor for thyroid cancer. The vast majority of radiation-induced cancers are papillary carcinomas (PTCs). These tumors typically have gene fusions in contrast to point mutations prevalent in sporadic PTCs. The aim of this study was to investigate the molecular profiles of PTC patients with workplace exposure to ionizing radiation.
Methods
A retrospective review of 543 patients who underwent surgery with diagnosis of PTC was performed. A cohort of nine healthcare specialists previously exposed to radiation sources during their professional practice was selected and analyzed using the ThyroSeq mutation panel for point mutations and gene fusions associated with thyroid cancer.
Results
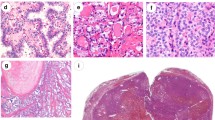
The molecular analysis of surgical samples of PTCs was informative and revealed genetic alterations in five patients. BRAF V600E was found in four (67%) cases whereas RET/PTC1 fusion in one (17%) and one sample (17%) was wild type for point mutations and fusions. One sample completely failed molecular analysis while two others were negative for genes fusions but failed DNA analysis; these three samples were excluded.
Conclusions
In this limited cohort of healthcare workers exposed to low dose of ionizing radiation at the workplace and developed PTC, the molecular profiling determined BRAF V600E point mutation as the most common event, arguing against the role of workplace radiation exposure in the etiology of these tumors.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2019. CA Cancer J. Clin. 69(1), 7–34 (2019)
American Cancer Society, Cancer Facts & Figures 2019. (Atlanta, GA: American Cancer Society, 2019)
V. Nose, Familial thyroid cancer: a review. Mod. Pathol. 24(Suppl 2), S19–S33 (2011)
C.M. Kitahara, J.A. Sosa, The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 12(11), 646–653 (2016)
J.A. Sipos, E.L. Mazzaferri, Thyroid cancer epidemiology and prognostic variables. Clin. Oncol. 22(6), 395–404 (2010)
J. Ferlay et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136(5), E359–E386 (2015)
M.T. Islam, Radiation interactions with biological systems. Int. J. Radiat. Biol. 93(5), 487–493 (2017)
V.A. LiVolsi et al. The Chernobyl thyroid cancer experience: pathology. Clin. Oncol. 23(4), 261–267 (2011)
M. Tronko et al. Radiation induced thyroid cancer: fundamental and applied aspects. Exp. Oncol. 32(3), 200–204 (2010)
E.S. Gilbert, Ionising radiation and cancer risks: what have we learned from epidemiology? Int. J. Radiat. Biol. 85(6), 467–482 (2009)
Y. Nikiforov, D.R. Gnepp, Pediatric thyroid cancer after the Chernobyl disaster. Pathomorphologic study of 84 cases (1991-1992) from the Republic of Belarus. Cancer 74(2), 748–766 (1994)
B.J. Duffy Jr, P.J. Fitzgerald, Thyroid cancer in childhood and adolescence; a report on 28 cases. Cancer 3(6), 1018–1032 (1950)
E.L. Socolow et al. Thyroid carcinoma in man after exposure to ionizing radiation. A summary of the findings in Hiroshima and Nagasaki. N. Engl. J. Med. 268, 406–410 (1963)
J.W. Wood et al. Thyroid carcinoma in atomic bomb survivors Hiroshima and Nagasaki. Am. J. Epidemiol. 89(1), 4–14 (1969)
D.R. Hollingsworth, Radiation and carcinoma of the thyroid. Conn. Med. 27, 762–765 (1963)
K. Takahashi et al. The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol. Carcinog. 46(3), 242–248 (2007)
K. Hamatani et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 68(17), 7176–7182 (2008)
E. Ron et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat. Res 141(3), 259–277 (1995)
L.H. Veiga et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat. Res. 185(5), 473–484 (2016)
Y. Nikiforov, D.R. Gnepp, J.A. Fagin, Thyroid lesions in children and adolescents after the Chernobyl disaster: implications for the study of radiation tumorigenesis. J. Clin. Endocrinol. Metab. 81(1), 9–14 (1996)
F. Pacini et al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J. Clin. Endocrinol. Metab. 82(11), 3563–3569 (1997)
L.M. Morton et al. Radiation-related genomic profile of papillary thyroid carcinoma after the Chernobyl accident. Sci 2021. 372(6543)
E. Cardis et al. Risk of thyroid cancer after exposure to 131I in childhood. J. Natl Cancer Inst. 97(10), 724–732 (2005)
D. Williams, Cancer after nuclear fallout: lessons from the Chernobyl accident. Nat. Rev. Cancer 2(7), 543–549 (2002)
E.K. Cahoon et al. Risk of thyroid nodules in residents of belarus exposed to chernobyl fallout as children and adolescents. J. Clin. Endocrinol. Metab. 102(7), 2207–2217 (2017)
A.B. Schneider et al. Dose-response relationships for radiation-induced thyroid cancer and thyroid nodules: evidence for the prolonged effects of radiation on the thyroid. J. Clin. Endocrinol. Metab. 77(2), 362–369 (1993)
M.J. Adams et al. Thyroid cancer risk 40+ years after irradiation for an enlarged thymus: an update of the Hempelmann cohort. Radiat. Res. 174(6), 753–762 (2010)
Y.E. Nikiforov, Radiation-induced thyroid cancer: what we have learned from chernobyl. Endocr. Pathol. 17(4), 307–317 (2006)
Y.E. Nikiforov, Is ionizing radiation responsible for the increasing incidence of thyroid cancer? Cancer 116(7), 1626–1628 (2010)
Cancer Genome Atlas Research, N., Integrated genomic characterization of papillary thyroid carcinoma. Cell 159(3), 676–690 (2014)
R.J. Leeman-Neill et al. RET/PTC and PAX8/PPARgamma chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer 119(10), 1792–1799 (2013)
J.C. Ricarte-Filho et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J. Clin. Invest. 123(11), 4935–4944 (2013)
R. Ciampi et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Invest. 115(1), 94–101 (2005)
A.A. Efanov et al. Investigation of the relationship between radiation dose and gene mutations and fusions in post-chernobyl thyroid cancer. J. Natl Cancer Inst. 110(4), 371–378 (2018)
R.J. Leeman-Neill et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 120(6), 799–807 (2014)
V. Lope et al. Occupation and thyroid cancer risk in Sweden. J. Occup. Environ. Med. 47(9), 948–957 (2005)
E. Albi, et al., Radiation and thyroid cancer. Int J Mol. Sci. 18(5) (2017)
J.X. Wang et al. Cancer incidence among medical diagnostic X-ray workers in China, 1950 to 1985. Int. J. Cancer 45(5), 889–895 (1990)
G. Wingren et al. Occupation and female papillary cancer of the thyroid. J. Occup. Environ. Med. 37(3), 294–297 (1995)
R, L., WHO classification of tumors of endocrine organs. 2017
Y.E. Nikiforov et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer 120(23), 3627–3634 (2014)
Y.E. Nikiforov et al. Impact of the multi-gene thyroseq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid 25(11), 1217–1223 (2015)
S. Leslie, G. Mary, W. Christian, TNM Classification of Malignant Tumors, 7th Edition. (New Jersey, 2011)
M.L. Iglesias et al. Radiation exposure and thyroid cancer: a review. Arch. Endocrinol. Metab. 61(2), 180–187 (2017)
L.T. Dilas et al. [Iodine and thyroid gland with or without nuclear catastrophe]. Med. Pregl. 65(11–12), 489–495 (2012)
Y.E. Nikiforov et al. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 57(9), 1690–1694 (1997)
H.M. Rabes et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res. 6(3), 1093–1103 (2000)
L. Fugazzola et al. Oncogenic rearrangements of the RET proto-oncogene in papillary thyroid carcinomas from children exposed to the Chernobyl nuclear accident. Cancer Res. 55(23), 5617–5620 (1995)
M.N. Nikiforova et al. Low prevalence of BRAF mutations in radiation-induced thyroid tumors in contrast to sporadic papillary carcinomas. Cancer Lett. 209(1), 1–6 (2004)
L. Luis, S. Augusto, Digital Imaging System for Plain Radiography. (New York, Springer, 2013)
S. Preston-Martin, B.E. Henderson, L. Bernstein, Medical and dental x rays as risk factors for recently diagnosed tumors of the head. Natl Cancer Inst. Monogr. 69, 175–179 (1985)
S. Preston-Martin et al. Prior exposure to medical and dental X-rays related to tumors of the parotid gland. J. Natl Cancer Inst. 80(12), 943–949 (1988)
W.T. Longstreth Jr et al. Dental X-rays and the risk of intracranial meningioma: a population-based case-control study. Cancer 100(5), 1026–1034 (2004)
A. Memon et al. Dental X-rays and the risk of thyroid cancer and meningioma: a systematic review and meta-analysis of current epidemiological evidence. Thyroid 29(11), 1572–1593 (2019)
M.A. Han, J.H. Kim, Diagnostic X-ray exposure and thyroid cancer risk: systematic review and meta-analysis. Thyroid 28(2), 220–228 (2018)
A. Hallquist et al. Medical diagnostic and therapeutic ionizing radiation and the risk for thyroid cancer: a case-control study. Eur. J. Cancer Prev. 3(3), 259–267 (1994)
P.D. Inskip et al. Medical diagnostic x rays and thyroid cancer. J. Natl Cancer Inst. 87(21), 1613–1621 (1995)
A. Bounacer et al. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene 15(11), 1263–1273 (1997)
Author information
Authors and Affiliations
Contributions
Y.E.N. and C.S.D. designed and developed the study. A.V., J.C., F.J., J.P.D., M.A., helped with samples collection. V.C. carried out the experiments, data analysis, and wrote the article with support from Y.E.N. and M.N.N. All authors participated in the revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki and its later amendments, and it was approved by the Institutional Review Board from both Hospital Pablo Tobón Uribe and Clínica las Americas, in Medellin, Colombia.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duque, C.S., Vélez, A., Cuartas, J. et al. Molecular profiling of papillary thyroid carcinomas in healthcare workers exposed to low dose radiation at the workplace. Endocrine 76, 95–100 (2022). https://doi.org/10.1007/s12020-021-02972-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02972-y