Abstract
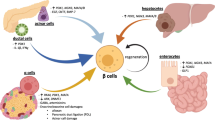
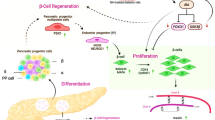
In both type 1 diabetes (T1D) and type 2 diabetes (T2D), there is a substantial β-cell mass loss. Residual β-cell mass is susceptible to cellular damage because of specific pancreatic β-cell characteristics. β cells have a low proliferation rate, being in human adults almost zero and a low antioxidant system that makes β cells susceptible to oxidative stress and increases their vulnerability to cell destruction. Different strategies have been addressed to preserve pancreatic β-cell residual mass and function in patients with diabetes. However, the effect of many compounds proposed in rodent models to trigger β-cell replication has different results in human β cells. In this review, scientific evidence of β-cell of two major regenerative approaches has been gathered. Regeneration proceedings for pancreatic β cells are promising and could improve β-cell proliferation capacity and contribute to the conservation of mature β-cell phenotypic characteristics. This evidence supports the notion that regenerative medicine could be a helpful strategy to yield amelioration of T1D and T2D pathogenesis.

Similar content being viewed by others
References
A. Pisania, G.C. Weir, J.J. O’Neil, A. Omer, V. Tchipashvili, J. Lei et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Investig. 90, 1661–75 (2011). https://doi.org/10.1038/labinvest.2010.124.Quantitative
H.I. Marrif, S.I. Al-Sunousi, Pancreatic β cell mass death. Front. Pharmacol. 7, 83 (2016). https://doi.org/10.3389/fphar.2016.00083
B.E. Gregg, P.C. Moore, D. Demozay, B.A. Hall, M. Li, A. Husain et al. Formation of a human β-cell population within pancreatic islets is set early in life. J. Clin. Endocrinol. Metab. 97, 3197–206 (2012). https://doi.org/10.1210/jc.2012-1206
I. Cozar-Castellano, N. Fiaschi-Taesch, T.A. Bigatel, K.K. Takane, A. García-Ocaña, R. Vasavada et al. Molecular control of cell cycle progression in the pancreatic β-cell. Endocr. Rev. 27, 356–70 (2006). https://doi.org/10.1210/er.2006-0004
Y. Dor, J. Brown, O.I. Martinez, D.A. Melton, Adult pancreatic β-cell are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–6 (2004)
I. Swenne, Effects of aging on the regenerative capacity of the pancreatic β-cell of the rat. Diabetes 32, 14–9 (1983)
R.N. Kulkarni, E.-B. Mizrachi, A. García-Ocaña, A.F. Stewart, Human β-cell proliferation and intracellular signaling driving in the dark without a road map. Diabetes 61, 2205–13 (2012). https://doi.org/10.2337/db12-0018
T. Mezza, R.N. Kulkarni, The regulation of pre- and post-maturational plasticity of mammalian islet cell mass. Diabetologia 57, 1291–303 (2014). https://doi.org/10.1007/s00125-014-3251-7
N. Fiaschi-Taesch, T.A. Bigatel, B. Sicari, K.K. Takane, F. Salim, S. Velazquez-Garcia et al. Survey of the human pancreatic β-cell G1/S proteome reveals a potential therapeutic role for Cdk-6 and cyclin D1 in enhancing human β-cell replication and function in vivo. Diabetes 58, 882–93 (2009). https://doi.org/10.2337/db08-0631.N.F.-T
L.U.C. Bouwens, I. Rooman, Regulation of pancreatic beta-cell mass. Physiol. Rev. 85, 1255–70 (2005). https://doi.org/10.1152/physrev.00025.2004
B. Reusens, C. Remacle, Programming of the endocrine pancreas by the early nutritional environment. Int J. Biochem Cell Biol. 38, 913–22 (2006). https://doi.org/10.1016/j.biocel.2005.10.012
D.J. Hill, Development of the endocrine pancreas. Rev. Endocr. Metab. Disord. 6, 229–38 (2005). https://doi.org/10.1016/B978-0-323-18907-1.00030-5
Q. Zhou, D.A. Melton, Pancreas regeneration. Nature 557, 351–8 (2018). https://doi.org/10.1038/s41586-018-0088-0
T.M. Nordmann, E. Dror, F. Schulze, S. Traub, E. Berishvili, C. Barbie et al. The role of inflammation in β-cell dedifferentiation. Sci. Rep. 2017:6285. https://doi.org/10.1038/s41598-017-06731-w.
C. Ackeifi, P. Wang, E. Karakose, J.E.M. Fox, B.J. González, H. Liu et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human β-cell regeneration. Sci. Transl. Med 12, eaaw9996 (2020)
D. Saunders, A.C. Powers, Replicative capacity of β-cells and type 1 diabetes. J. Autoimmun. 71, 59–68 (2016). https://doi.org/10.1016/j.jaut.2016.03.014
E. Dirice, D. Walpita, A. Vetere, B.C. Meier, S. Kahraman, J. Hu et al. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes 65, 1660–71 (2016). https://doi.org/10.2337/db15-1127
C. Ackeifi, E. Swartz, K. Kumar, H. Liu, S. Chalada, E. Karakose et al. Pharmacologic and genetic approaches define human pancreatic β cell mitogenic targets of DYRK1A inhibitors. JCI Insight 5, e132594 (2020)
P. Wang, E. Karakose, H. Liu, E. Swartz, V. Zlatanic, J. Wilson et al. Combined inhibition of DYRK1A, SMAD and trithorax pathways synergizes to induce robust replication in adult human beta cells. Cell Metab. 29, 638–52 (2020). https://doi.org/10.1016/j.cmet.2018.12.005.Combined
P. Wang, J. Alvarez-Perez, D.P. Felsenfeld, S. Sivendran, A. Bender, A. Kumar et al. Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase. Nat. Med. 21, 383–8 (2015). https://doi.org/10.1038/nm.3820.Induction
E. Karakose, C. Ackeifi, P. Wang, A.F. Stewart, Advances in drug discovery for human beta cell regeneration. Diabetologia 61, 1693–9 (2018). https://doi.org/10.1007/s00125-018-4639-6
Y. Gwack, S. Sharma, J. Nardone, B. Tanasa, A. Iuga, S. Srikanth et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441, 646–50 (2006). https://doi.org/10.1038/nature04631
J. Shirakawa, R.N. Kulkarni, Novel factors modulating human β-cell proliferation. Diabetes Obes. Metab. 18, 71–7 (2017). https://doi.org/10.1111/dom.12731.Novel
W.R. Goodyer, X. Gu, Y. Liu, R. Bottino, G.R. Crabtree, S.K. Kim, Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT. Dev. Cell 23, 21–34 (2012). https://doi.org/10.1016/j.devcel.2012.05.014.Neonatal
W. Shen, B. Taylor, Q. Jin, S. Meeusen, Y. Zhang, A. Kamireddy et al. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat. Commun. 6, 8372 (2015). https://doi.org/10.1038/ncomms9372
K.I. Aamodt, R. Aramandla, J.J. Brown, N. Fiaschi-Taesch, P. Wang, A.F. Stewart et al. Development of a reliable automated screening system to identify small molecules and biologics that promote human β-cell regeneration. Am. J. Physiol. Endocrinol. Metab. 311, E859–68 (2016). https://doi.org/10.1152/ajpendo.00515.2015
S. Dhawan, E. Dirice, R.N. Kulkarni, A. Bhushan, Inhibition of TGF-β signaling promotes human pancreatic β-cell replication. Diabetes 65, 1208–18 (2016). https://doi.org/10.2337/db15-1331
G. Basile, R.N. Kulkarni, N.G. Morgan, How, when, and where do human β-cells regenerate? Curr. Diab Rep. 19, 48 (2020). https://doi.org/10.1007/s11892-019-1176-8.How
Y. Nakagawa, T. Suzuki, H. Ishii, A. Ogata, D. Nakae, Mitochondrial dysfunction and biotransformation of β-carboline alkaloids, harmine and harmaline, on isolated rat hepatocytes. Chem. Biol. Interact. 188, 393–403 (2010). https://doi.org/10.1016/j.cbi.2010.09.004
H. Waki, K.W. Park, N. Mitro, L. Pei, R. Damoiseaux, D.C. Wilpitz et al. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPAR g expression. Cell Metab. 5, 357–70 (2007). https://doi.org/10.1016/j.cmet.2007.03.010
B. Khor, J.D. Gagnon, G. Goel, M.I. Roche, K.L. Conway, K. Tran et al. The kinase DYRK1A reciprocally regulates the differentiation of Th17 and regulatory T cells. Elife 4, e05920 (2015). https://doi.org/10.7554/eLife.05920
D. Kondoh, S. Yamamoto, T. Tomita, K. Miyazaki, N. Itoh, Y. Yasumoto et al. Harmine lengthens circadian period of the mammalian molecular clock in the suprachiasmatic nucleus. Biol. Pharm. Bull. 37, 1422–7 (2014)
H.E. Hohmeier, L. Zhang, B. Taylor, S. Stephens, D. Lu, P. Mcnamara et al. Identification of a small molecule that stimulates human β-cell proliferation and insulin secretion, and protects against cytotoxic stress in rat insulinoma cells. PLoS ONE 15, e0224344 (2020). https://doi.org/10.1371/journal.pone.0224344
Y. Li, B. Hao, Structural basis of dimerization-dependent ubiquitination by the SCF Fbx4 ubiquitin ligase. J. Biol. Chem. 285, 13896–906 (2010). https://doi.org/10.1074/jbc.M110.111518
S. Tiwari, C. Roel, T. Mansoor, R. Wills, N. Perianayagam, P. Wang et al. Definition of a Skp2-c-Myc Pathway to Expand Human Beta-cells. Sci. Rep. 6, 28461 (2016). https://doi.org/10.1038/srep28461
P.P. Khin, J.H. Lee, H.S. Jun, A brief review of the mechanisms of β-cell dedifferentiation in type 2 diabetes. Nutrients 2021;13. https://doi.org/10.3390/nu13051593.
O. Friedman-Mazursky, R. Elkon, S. Efrat, Redifferentiation of expanded human islet β cells by inhibition of ARX. Sci. Rep. 6, 20698 (2016). https://doi.org/10.1038/srep20698
J. Zhang, F. Liu, The De-, Re-, and trans-differentiation of β-cells: Regulation and function. Semin Cell Dev. Biol. 103, 68–75 (2020). https://doi.org/10.1016/j.semcdb.2020.01.003
G. Domínguez Gutiérrez, A.S. Bender, V. Cirulli, T.L. Mastracci, S.M. Kelly, A. Tsirigos et al. Pancreatic β cell identity requires continual repression of non–β cell programs. J. Clin. Investig 127, 244–59 (2017). https://doi.org/10.1172/JCI88017.NEUROD1
M. Bensellam, J.C. Jonas, D.R. Laybutt, Mechanisms of β-cell dedifferentiation in diabetes: Recent findings and future research directions. J. Endocrinol. 236, R109–43 (2018). https://doi.org/10.1530/JOE-17-0516
Sachs S., Bastidas-Ponce A., Tritschler S., Bakhti M., Böttcher A., Sánchez-Garrido M.A., et al. Targeted Pharmacological Therapy Restores β-Cell Function for Diabetes Remission. 2 (Springer US, 2020). https://doi.org/10.1038/s42255-020-0171-3.
W. Zhiyu, N.W. York, C.G. Nichols, M.S. Remedi, Pancreatic β-cell dedifferentiation in diabetes and re-differentiation following insulin therapy. Cell Metab. 19, 872–82 (2014). https://doi.org/10.1016/j.cmet.2014.03.010.Pancreatic
X.N. Téllez, M. Vilaseca, Y. Martí, A. Pla, E. Montanya, β-Cell dedifferentiation, reduced duct cell plasticity, and impaired β-cell mass regeneration in middle-aged rats. Am. J. Physiol. Endocrinol. Metab. 311, E554–63 (2016). https://doi.org/10.1152/ajpendo.00502.2015
C. Talchai, S. Xuan, H.V. Lin, L. Sussel, D. Accili, Pancreatic β-cell dedifferentiation as mechanism of diabetic β-cell failure. Cell 150, 1223–34 (2012). https://doi.org/10.1016/j.cell.2012.07.029.Pancreatic
Y. Bar, H.A. Russ, S. Knoller, L. Ouziel-Yahalom, E. Shimon, HES-1 is involved in adaptation of adult human β-cells to proliferation in vitro. Diabetes 57, 2413–20 (2008). https://doi.org/10.2337/db07-1323
S. Efrat, Mechanisms of adult human β-cell in vitro dedifferentiation and redifferentiation. Diabetes Obes. Metab. 18, 97–101 (2016). https://doi.org/10.1111/dom.12724
A. Lenz, G. Toren-Haritan, S. Efrat, Redifferentiation of adult human β cells expanded in vitro by inhibition of the WNT pathway. PLoS ONE 9, e112914 (2014). https://doi.org/10.1371/journal.pone.0112914
M. Bakhti, A. Böttcher, H. Lickert, Modelling the endocrine pancreas in health and disease. Nat. Rev. Endocrinol. 15, 155–71 (2019). https://doi.org/10.1038/s41574-018-0132-z
W.L. Qiu, Y.W. Zhang, Y. Feng, L.C. Li, L. Yang, C.R. Xu, Deciphering pancreatic islet β cell and α cell maturation pathways and characteristic features at the single-cell level. Cell Metab. 25, 1194–1205.e4 (2017). https://doi.org/10.1016/j.cmet.2017.04.003
C. Zeng, F. Mulas, Y. Sui, T. Guan, N. Miller, F. Liu et al. Pseudotemporal ordering of single cells reveals metabolic control of postnatal beta-cell proliferation. Cell Metab. 25, 1160–75 (2017).https://doi.org/10.1016/j.cmet.2017.04.014.Pseudotemporal
J. Sun, Q. Ni, J. Xie, M. Xu, J. Zhang, J. Kuang et al. β-cell dedifferentiation in patients With T2D With adequate glucose control and nondiabetic chronic pancreatitis. J. Clin. Endocrinol. Metab. 104, 83–94 (2019). https://doi.org/10.1210/jc.2018-00968
W. Ying, Y.S. Lee, Y. Dong, J.S. Seidman, M. Yang, R. Isaac et al. Expansion of islet-resident macrophages leads to inflammation affecting β cell proliferation and function in obesity. Cell Metab. 29, 457–474.e5 (2019). https://doi.org/10.1016/j.cmet.2018.12.003
W. He, T. Yuan, K. Maedler, Macrophage-associated pro-inflammatory state in human islets from obese individuals. Nutr. Diabetes 9, 36 (2019). https://doi.org/10.1038/s41387-019-0103-z
M. Ghodsi, B. Larijani, A.A. Keshtkar, E. Nasli-Esfahani, S. Alatab, M.R. Mohajeri-Tehrani, Mechanisms involved in altered bone metabolism in diabetes: a narrative review. J. Diabetes Metab. Disord. 15, 52 (2016). https://doi.org/10.1186/s40200-016-0275-1
R.A. DeFronzo, E. Ferrannini, L. Groop, R.R. Henry, W.H. Herman, J.J. Holst et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 1, 1–22 (2015). https://doi.org/10.1038/s41574-019-0286-3
S. Efrat, Beta-cell dedifferentiation in type 2 diabetes: concise review. Transl. Clin. Res. 37, 1267–72 (2019). https://doi.org/10.1002/stem.3059
F. Han, X. Li, J. Yang, H. Liu, Y. Zhang, X. Yang et al. Salsalate prevents β-cell dedifferentiation in OLETF rats with type 2 diabetes through Notch1 pathway. Aging Dis. 10, 719–30 (2019). https://doi.org/10.14336/AD.2018.1221
Y.S. Oh, S. Shin, Y.J. Lee, E.H. Kim, H.S. Jun, Betacellulin-induced beta cell proliferation and regeneration is mediated by activation of ErbB-1 and ErbB-2 receptors. PLoS ONE. 2011;6. https://doi.org/10.1371/journal.pone.0023894.
S. Shin, N. Li, N. Kobayashi, J.W. Yoon, H.S. Jun, Remission of diabetes by β-cell regeneration in diabetic mice treated with a recombinant adenovirus expressing Betacellulin. Mol. Ther. 16, 854–61 (2008). https://doi.org/10.1038/mt.2008.22
H. Kojima, M. Fujimiya, K. Matsumura, P. Younan, H. Imaeda, M. Maeda et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 9, 596–603 (2003). https://doi.org/10.1038/nm867
L. Li, M. Seno, H. Yamada, I. Kojima, Betacellulin improves glucose metabolism by promoting conversion of intraislet precursor cells to β-cells in streptozotocin-treated mice. Am. J. Physiol. Endocrinol. Metab. 285, 577–83 (2003). https://doi.org/10.1152/ajpendo.00120.2003
L. Ouziel-Yahalom, M. Zalzman, L. Anker-kitai, S. Knoller, Y. Bar, M. Glandt et al. Expansion and redifferentiation of adult human pancreatic islet cells. Biochem. Biophys. Res. Commun. 341, 291–8 (2006). https://doi.org/10.1016/j.bbrc.2005.12.187
Y.S. Lee, G.J. Song, H.S. Jun, Betacellulin-induced α-cell proliferation is mediated by ErbB3 and ErbB4, and may contribute to β-cell regeneration. Front Cell Dev. Biol. 8, 1–11 (2021). https://doi.org/10.3389/fcell.2020.605110
M.Y. Song, U.J. Bae, K.Y. Jang, B.H. Park, Transplantation of betacellulin-transduced islets improves glucose intolerance in diabetic mice. Exp. Mol. Med. 46, e98–8 (2014). https://doi.org/10.1038/emm.2014.24
H.A. Russ, E. Sintov, L. Anker-Kitai, O. Friedman, A. Lenz, G. Toren et al. Insulin-producing cells generated from dedifferentiated human pancreatic beta cells expanded in vitro. PLoS ONE 6, e25566 (2011). https://doi.org/10.1371/journal.pone.0025566
R.P. Robertson, L.K. Olson, H.-J. Zhang, Differentiating glucose toxicity from glucose desensitization: A new message from the insulin gene. Diabetes 43, 1085–9 (1994). https://doi.org/10.2337/diab.43.9.1085
E. Sintov, G. Nathan, S. Knoller, M. Pasmanik-Chor, H.A. Russ, E. Shimon, Inhibition of ZEB1 expression induces redifferentiation of adult human β cells expanded in vitro. Sci. Rep. 5, 13024 (2015). https://doi.org/10.1038/srep13024
B. Finan, B. Yang, N. Ottaway, K. Stemmer, T.D. Müller, C.-X. Yi et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 18, 1847–56 (2012). https://doi.org/10.1038/nm.3009.Targeted
F. Mauvais-Jarvis, C. Le May, J.P. Tiano, S. Liu, G. Kilic-Berkmen, J.H. Kim, The role of estrogens in pancreatic islet physiopathology. Adv. Exp. Med Biol. 1043, 385–99 (2017). https://doi.org/10.1007/978-3-319-70178-3_18
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Desentis-Desentis, M.F. Regenerative approaches to preserve pancreatic β-cell mass and function in diabetes pathogenesis. Endocrine 75, 338–350 (2022). https://doi.org/10.1007/s12020-021-02941-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02941-5