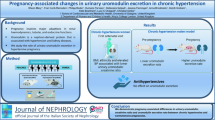
Abstract
Background
During pregnancy, the renin–angiotensin–aldosterone system (RAAS) undergoes major changes to preserve normal blood pressure (BP) and placental blood flow and to ensure a good pregnancy outcome. Abnormal aldosterone–renin metabolism is a risk factor for arterial hypertension and cardiovascular risk, but its association with pathological conditions in pregnancy remains unknown. Moreover, potential biomarkers associated with these pathological conditions should be identified.
Aim
To study a cohort of normotensive pregnant women according to their serum aldosterone and plasma renin levels and assay their small extracellular vesicles (sEVs) and a specific protein cargo (LCN2, AT1R).
Methods
A cohort of 54 normotensive pregnant women at term gestation was included. We determined the BP, serum aldosterone, and plasma renin concentrations. In a subgroup, we isolated their plasma sEVs and semiquantitated two EV proteins (AT1R and LCN2).
Results
We set a normal range of aldosterone and renin based on the interquartile range. We identified 5/54 (9%) pregnant women with elevated aldosterone and low renin levels and 5/54 (9%) other pregnant women with low aldosterone and elevated renin levels. No differences were found in sEV-LCN2 or sEV-AT1R.
Conclusion
We found that 18% of normotensive pregnant women had either high aldosterone or high renin levels, suggesting a subclinical status similar to primary aldosteronism or hyperreninemia, respectively. Both could evolve to pathological conditions by affecting the maternal vascular and renal physiology and further the BP. sEVs and their specific cargo should be further studied to clarify their role as potential biomarkers of RAAS alterations in pregnant women.


Similar content being viewed by others
References
E.R. Lumbers, K.G. Pringle, Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306(2), R91–101 (2014). https://doi.org/10.1152/ajpregu.00034.2013
C. Gennari-Moser, G. Escher, S. Kramer, B. Dick, N. Eisele, M. Baumann, et al. Normotensive blood pressure in pregnancy: the role of salt and aldosterone. Hypertension 63(2), 362–8 (2014). https://doi.org/10.1161/HYPERTENSIONAHA.113.02320
E. Landau, L. Amar, Primary aldosteronism and pregnancy. Ann. Endocrinol. 77(2), 148–60 (2016). https://doi.org/10.1016/j.ando.2016.04.009
P. Soma-Pillay, C. Nelson-Piercy, H. Tolppanen, A. Mebazaa, Physiological changes in pregnancy. Cardiovasc J. Afr. 27(2), 89–94 (2016). https://doi.org/10.5830/CVJA-2016-021
A.H. Affinati, R.J. Auchus, Endocrine causes of hypertension in pregnancy. Gland Surg. 9(1), 69–79 (2020). https://doi.org/10.21037/gs.2019.12.04
M.A. Sparks, S.D. Crowley, S.B. Gurley, M. Mirotsou, T.M. Coffman, Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 4(3), 1201–1228 (2014). https://doi.org/10.1002/cphy.c130040
A. Nguyen Dinh Cat, A.C. Montezano, D. Burger, R.M. Touyz, Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 19(10), 1110–20 (2013). https://doi.org/10.1089/ars.2012.4641
C. Savoia, D. Burger, N. Nishigaki, A. Montezano, R.M. Touyz, Angiotensin II and the vascular phenotype in hypertension. Expert Rev. Mol. Med. 13, e11 (2011). https://doi.org/10.1017/S1462399411001815
L. Thapa, C.M. He, H.P. Chen, Study on the expression of angiotensin II (ANG II) receptor subtype 1 (AT1R) in the placenta of pregnancy-induced hypertension. Placenta 25(7), 637–41 (2004). https://doi.org/10.1016/j.placenta.2004.01.026
P.J. Williams, H.D. Mistry, B.A. Innes, J.N. Bulmer, F. Broughton Pipkin, Expression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the human. Placenta 31(5), 448–55 (2010). https://doi.org/10.1016/j.placenta.2010.02.014
F.M. Rogerson, Y. Yao, B.J. Smith, P.J. Fuller, Differences in the determinants of eplerenone, spironolactone and aldosterone binding to the mineralocorticoid receptor. Clin. Exp. Pharmacol. Physiol. 31(10), 704–9 (2004). https://doi.org/10.1111/j.1440-1681.2004.04079.x
J.M.C. Connell, E. Davies, The new biology of aldosterone. J. Endocrinol. 186(1), 1 (2005). https://doi.org/10.1677/joe.1.06017
S. Alvarez, C. Suazo, A. Boltansky, M. Ursu, D. Carvajal, G. Innocenti, et al. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant. Proc. 45(10), 3719–23 (2013). https://doi.org/10.1016/j.transproceed.2013.08.079
P.J. Fuller, Y.Z. Yao, R. Jin, S. He, B. Martin-Fernandez, M.J. Young, et al. Molecular evolution of the switch for progesterone and spironolactone from mineralocorticoid receptor agonist to antagonist. Proc. Natl Acad. Sci. USA 116(37), 18578–83 (2019). https://doi.org/10.1073/pnas.1903172116
M.E. Baker, Y. Katsu, Progesterone: an enigmatic ligand for the mineralocorticoid receptor. Biochem. Pharmacol. 177, 113976 (2020). https://doi.org/10.1016/j.bcp.2020.113976
A. Vecchiola, C.F. Lagos, C.A. Fuentes, F. Allende, C. Campino, C. Valdivia, et al. Different effects of progesterone and estradiol on chimeric and wild type aldosterone synthase in vitro. Reprod. Biol. Endocrinol. 11, 76 (2013). https://doi.org/10.1186/1477-7827-11-76
C. Campino, P. Trejo, C.A. Carvajal, A. Vecchiola, C. Valdivia, C.A. Fuentes, et al. Pregnancy normalized familial hyperaldosteronism type I: a novel role for progesterone? J. Hum. Hypertens. 29(2), 138–9 (2015). https://doi.org/10.1038/jhh.2014.49
C. Latouche, S. El Moghrabi, S. Messaoudi, A. Nguyen Dinh Cat, I. Hernandez-Diaz, D. Alvarez de la Rosa, et al. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension 59(5), 966–72 (2012). https://doi.org/10.1161/HYPERTENSIONAHA.111.187872
A. Tarjus, E. Martinez-Martinez, C. Amador, C. Latouche, S. El Moghrabi, T. Berger, et al. Neutrophil gelatinase-associated lipocalin, a novel mineralocorticoid biotarget, mediates vascular profibrotic effects of mineralocorticoids. Hypertension 66(1), 158–66 (2015). https://doi.org/10.1161/HYPERTENSIONAHA.115.05431
C. Thery, K.W. Witwer, Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7(1), 1535750 (2018). https://doi.org/10.1080/20013078.2018.1535750
C. Salomon, G.E. Rice, Chapter six–role of exosomes in placental homeostasis and pregnancy disorders. In Progress in Molecular Biology and Translational Science, vol. 145, ed. by W.R. Huckle (Academic Press; Amsterdam, The Netherlands 2017), 163–179
M.D. Mitchell, H.N. Peiris, M. Kobayashi, Y.Q. Koh, G. Duncombe, S.E. Illanes, et al. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 213(4, Supplement), S173–S81 (2015). https://doi.org/10.1016/j.ajog.2015.07.001
C. Salomon, M.J. Torres, M. Kobayashi, K. Scholz-Romero, L. Sobrevia, A. Dobierzewska, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS ONE 9(6), e98667 (2014). https://doi.org/10.1371/journal.pone.0098667
X.-B.L. Hong-Chao Zhang, S. Huang, X.-Y. Bi, H.-X. Wang, L.-X. Xie, Y.-Q. Wang, X.-F. Cao, J. Lv, F.-J. Xiao, Y. Yang, Z.-K. Guo, Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 21(18), 3289–97 (2012). https://doi.org/10.1089/scd.2012.0095
E.R.P.P. Carrillo, Lipocalina asociada con la gelatinasa de neutrófilos. Un biomarcador que llegó para quedarse. Med. Crít 4, 258–63 (2014)
M. Laskowska, B. Leszczyńska-Gorzelak, J. Oleszczuk, Placental angiotensin II receptor AT1R in normotensive patients and its correlation between infant birth weight. Eur. J. Obstet. Gynecol. Reprod. Biol. 109(2), 166–70 (2003). https://doi.org/10.1016/S0301-2115(03)00079-4
P.J. Williams, H.D. Mistry, B.A. Innes, J.N. Bulmer, F. Broughton Pipkin, Expression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the human. Placenta 31(5), 448–55 (2010). https://doi.org/10.1016/j.placenta.2010.02.014
P.K. Whelton, R.M. Carey, W.S. Aronow, D.E. Casey, K.J. Collins, C.D. Himmelfarb, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71(6), e13–e115 (2018). https://doi.org/10.1161/HYP.0000000000000065
A. Markou, T. Pappa, G. Kaltsas, A. Gouli, K. Mitsakis, P. Tsounas, et al. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: a very high odds ratio for progression into arterial hypertension. J. Clin. Endocrinol. Metab. 98(4), 1409–16 (2013). https://doi.org/10.1210/jc.2012-3353
R. Baudrand, F.J. Guarda, C. Fardella, G. Hundemer, J. Brown, G. Williams, et al. Continuum of renin-independent aldosteronism in normotension. Hypertension 69(5), 950–6 (2017). https://doi.org/10.1161/HYPERTENSIONAHA.116.08952
C. Campino, C. Hill, R. Baudrand, A. Martinez-Aguayo, M. Aglony, C.A. Carrasco, et al. Usefulness and pitfalls in sodium intake estimation: comparison of dietary assessment and urinary excretion in chilean children and adults. Am. J. Hypertens. 29(10), 1212–7 (2016). https://doi.org/10.1093/ajh/hpw056. Epub 2016/06/10PubMed PMID: 27279009
A. Tapia-Castillo, R. Baudrand, A. Vaidya, C. Campino, F. Allende, C. Valdivia, et al. Clinical, biochemical, and genetic characteristics of "nonclassic" apparent mineralocorticoid excess syndrome. J. Clin. Endocrinol. Metab. 104(2), 595–603 (2019). https://doi.org/10.1210/jc.2018-01197
S. Sarker, K. Scholz-Romero, A. Perez, S.E. Illanes, M.D. Mitchell, G.E. Rice, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 12, 204 (2014). https://doi.org/10.1186/1479-5876-12-204
A. Tapia-Castillo, D. Guanzon, C. Palma, A. Lai, E. Barros, F. Allende, et al. Downregulation of exosomal miR-192-5p and miR-204-5p in subjects with nonclassic apparent mineralocorticoid excess. J. Transl. Med. 17(1), 392 (2019). https://doi.org/10.1186/s12967-019-02143-8
B. Fuenzalida, C. Cantin, S. Kallol, L. Carvajal, V. Pasten, S. Contreras-Duarte, et al. Cholesterol uptake and efflux are impaired in human trophoblast cells from pregnancies with maternal supraphysiological hypercholesterolemia. Sci. Rep. 10(1), 5264 (2020). https://doi.org/10.1038/s41598-020-61629-4
J.W. Funder, R.M. Carey, F. Mantero, M.H. Murad, M. Reincke, H. Shibata, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 101, 1889–916 (2016). https://doi.org/10.1210/jc.2015-4061
P. Mulatero, M. Stowasser, K.C. Loh, C.E. Fardella, R.D. Gordon, L. Mosso, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J. Clin. Endocrinol. Metab. 89(3), 1045–50 (2004). Epub 2004/03/06
Y. Takeda, S. Karashima, T. Yoneda, Primary aldosteronism, diagnosis and treatment in Japan. Rev. Endocr. Metab. Disord. 12(1), 21–5 (2011). https://doi.org/10.1007/s11154-011-9164-6
L. Mosso, C. Carvajal, A. Gonzalez, A. Barraza, F. Avila, J. Montero, et al. Primary aldosteronism and hypertensive disease. Hypertension 42(2), 161–5 (2003). https://doi.org/10.1161/01.HYP.0000079505.25750.11
K. Omata, S.A. Tomlins, W.E. Rainey, Aldosterone-producing cell clusters in normal and pathological states. Horm. Metab. Res 49(12), 951–6 (2017). https://doi.org/10.1055/s-0043-122394
A. Martinez-Aguayo, M. Aglony, C. Campino, H. Garcia, R. Bancalari, L. Bolte, et al. Aldosterone, plasma Renin activity, and aldosterone/renin ratio in a normotensive healthy pediatric population. Hypertension 56(3), 391–6 (2010). https://doi.org/10.1161/HYPERTENSIONAHA.110.155135
C.E. Fardella, L.M. Mosso, C.A. Carvajal, [Primary aldosteronism]. Rev. Med Chil. 136(7), 905–14 (2008)
W.A. Hsueh, J.A. Luetscher, E.J. Carlson, G. Grislis, E. Fraze, A. McHargue, Changes in active and inactive renin throughout pregnancy. J. Clin. Endocrinol. Metab. 54(5), 1010–6 (1982). https://doi.org/10.1210/jcem-54-5-1010
E.R. Lumbers, S.J. Delforce, A.L. Arthurs, K.G. Pringle, Causes and consequences of the dysregulated maternal renin-angiotensin system in preeclampsia. Front Endocrinol. 10, 563 (2019). https://doi.org/10.3389/fendo.2019.00563
A. Larsson, M. Palm, L.O. Hansson, O. Axelsson, Reference values for clinical chemistry tests during normal pregnancy. Bjog 115(7), 874–81 (2008). https://doi.org/10.1111/j.1471-0528.2008.01709.x
M.K. Kashyap, S.V. Saxena, M. Khullar, H. Sawhney, K. Vasishta, Role of anion gap and different electrolytes in hypertension during pregnancy (preeclampsia). Mol. Cell Biochem. 282(1-2), 157–67 (2006). https://doi.org/10.1007/s11010-006-1739-2
L. Lyu, H. Wang, B. Li, Q. Qin, L. Qi, M. Nagarkatti, et al. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J. Mol. Cell. Cardiol. 89, 268–79 (2015). https://doi.org/10.1016/j.yjmcc.2015.10.022
G. Pironti, R.T. Strachan, D. Abraham, S.M.-W. Yu, M. Chen, W. Chen, et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131(24), 2120–30 (2015). https://doi.org/10.1161/CIRCULATIONAHA.115.015687
E. Willms, H.J. Johansson, I. Mager, Y. Lee, K.E. Blomberg, M. Sadik, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6, 22519 (2016). https://doi.org/10.1038/srep22519
C.A. Carvajal, A. Tapia-Castillo, A. Vecchiola, R. Baudrand, C.E. Fardella, Classic and nonclassic apparent mineralocorticoid excess syndrome. J. Clin. Endocrinol. Metab. 105(4), dgz315 (2020). https://doi.org/10.1210/clinem/dgz315
Funding
This study was supported by the following grants: ANID/CONICYT-FONDECYT 1160695, 1190250, and 1212006; FONDECYT-POSTDOCTORAL 3200646; CONICYT-FONDEQUIP EQM150023; ANID–Millennium Science Initiative Program—IMII P09/016-F, ICN09_016; CORFO BMRC-13CTI-21526-P1; and SOCHED 2019-09 and CETREN-UC.
Author information
Authors and Affiliations
Contributions
V.P., A.L., C.A.C., C.E.F. and A.T.-C. designed the study, collected, analyzed, interpreted the patients data, and wrote the first draft of the manuscript. All authors contributed to the discussion, reviewed the manuscript, and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pastén, V., Tapia-Castillo, A., Fardella, C.E. et al. Aldosterone and renin concentrations were abnormally elevated in a cohort of normotensive pregnant women. Endocrine 75, 899–906 (2022). https://doi.org/10.1007/s12020-021-02938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02938-0