Abstract
Purpose
Circulating estrogens in breast cancer patients and survivors are often extremely low due to menopause and estrogen-reducing cancer treatments. Simultaneously, circulating inflammatory markers, and inflammatory proteins in brains of rodent tumor models, can be elevated and correlate with debilitating neurological and psychological comorbidities. Because estrogen has anti-inflammatory properties in the brain, we hypothesized that mammary tumor-induced neuroinflammation is driven, in part, by reduced brain estrogen signaling.
Methods
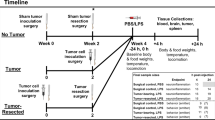
An ovariectomized mouse model of postmenopausal breast cancer utilizing the ERα-positive 67NR mammary tumor cell line was used for these experiments. A novel, orally bioavailable, and brain penetrant ERβ agonist was administered daily via oral gavage. Following treatment, estrogen-responsive genes were measured in brain regions. Central and circulating inflammatory markers were measured via RT-qPCR and a multiplex cytokine array, respectively.
Results
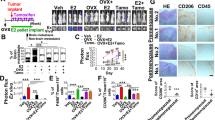
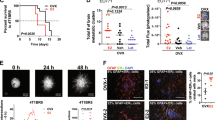
We present novel findings that peripheral mammary tumors alter estrogen signaling genes including receptors and aromatase in the hypothalamus, hippocampus, and frontal cortex. Mammary tumors induced peripheral and central inflammation, however, pharmacological ERβ activation was not sufficient to reduce this inflammation.
Conclusions
Data presented here suggest that compensating for low circulating estrogen with ERβ brain activation is not sufficient to attenuate mammary tumor-induced neuroinflammation, and is therefore not a likely candidate for the treatment of behavioral symptoms in patients. The novel finding that mammary tumors alter estrogen signaling-related genes is a clinically relevant advancement to the understanding of how peripheral tumor biology modulates neurobiology. This is necessary to predict and prevent behavioral comorbidities (e.g., cognitive impairment) prevalent in cancer patients and survivors.




Similar content being viewed by others
Data availability
Primary data will be made available upon request. For access to OSU-ERβ-12 please consult Dr. Christopher Coss of The Ohio State University, Comprehensive Cancer Center, Drug Development Institute.
References
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2020. CA Cancer J. Clin. (2020). https://doi.org/10.3322/caac.21590
L.M. Hess, K.C. Insel, Chemotherapy-related change in cognitive function: a conceptual model. Oncol. Nurs. Forum (2007). https://doi.org/10.1188/07.ONF.981-994
J.R. Satin, W. Linden, M.J. Phillips, Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer (2009). https://doi.org/10.1002/cncr.24561
M.C. Janelsins, K.M. Mustian, O.G. Palesh, S.G. Mohile, L.J. Peppone, L.K. Sprod, C.E. Heckler, J.A. Roscoe, A.W. Katz, J.P. Williams, G.R. Morrow, Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support. Care Cancer (2012). https://doi.org/10.1007/s00520-011-1158-0
M. Seretny, G.L. Currie, E.S. Sena, S. Ramnarine, R. Grant, MacLeod M.R., L.A. Colvin, M. Fallon, Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain (2014). https://doi.org/10.1016/j.pain.2014.09.020
A. Schrepf, S.K. Lutgendorf, L.M. Pyter, Pre-treatment effects of peripheral tumors on brain and behavior: Neuroinflammatory mechanisms in humans and rodents. Brain Behav. Immun. (2015). https://doi.org/10.1016/j.bbi.2015.04.010
R.M. Barrientos, L.D. Strehle, A.A. Lahoud, L.M. Pyter, Mammary tumors suppress aging-induced neuroinflammation in female Balb/c mice. Compr. Psychoneuroendocrinol. (2020). https://doi.org/10.1016/j.cpnec.2020.100002
L.M. Pyter, V. Pineros, J.A. Galang, M.K. McClintock, B.J. Prendergast, Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc. Natl. Acad. Sci. (2009). https://doi.org/10.1073/pnas.0811949106
N.L. Sparkman, J.B. Buchanan, J.R.R. Heyen, J. Chen, J.L. Beverly, R.W. Johnson, Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J. Neurosci. (2006). https://doi.org/10.1523/JNEUROSCI.3376-06.2006
D.M. Norden, J.P. Godbout, Review: Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. (2013). https://doi.org/10.1111/j.1365-2990.2012.01306.x
L. Pietranera, M.J. Bellini, M.A. Arévalo, R. Goya, M.E. Brocca, L.M. Garcia-Segura, A.F. De Nicola, Increased aromatase expression in the hippocampus of spontaneously hypertensive rats: effects of estradiol administration. Neuroscience (2011). https://doi.org/10.1016/j.neuroscience.2010.11.044
M.I. Rossberg, S.J. Murphy, R.J. Traystman, P.D. Hurn, LY353391.HCl, a Selective Estrogen Receptor Modulator and Experimental Stroke. Stroke (2000). https://doi.org/10.1161/01.str.31.12.3041
H.V.O. Carswell, A.F. Dominiczak, L.M. Garcia-Segura, N. Harada, J.B. Hutchinson, I.M. Macrae, Brain aromatase expression after experimental stroke: topography and time course. J. Steroid Biochem. Mol. Biol. (2005). https://doi.org/10.1016/j.jsbmb.2005.02.016
C.E. DeSantis, J. Ma, M.M. Gaudet, L.A. Newman, K.D. Miller, A.G. Sauer, A. Jemal, R.L. Siegel, Breast cancer statistics, 2019. CA Cancer J. Clin. (2019). https://doi.org/10.3322/caac.21583
X. Qian, Z. Li, G. Ruan, C. Tu, W. Ding, Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials. Breast Cancer Res. Treat. (2020). https://doi.org/10.1007/s10549-019-05464-w
H.K. Patel, T. Bihani, Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. (2018). https://doi.org/10.1016/J.PHARMTHERA.2017.12.012
W.M. Pardridge, L.J. Mietus, Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J. Clin. Investig. (1979). https://doi.org/10.1172/JCI109433
M. Bixo, T. Bäckström, B. Winblad, A. Andersson, Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J. Steroid Biochem. Mol. Biol. (1995). https://doi.org/10.1016/0960-0760(95)00179-4
C.M. Brown, T.A. Mulcahey, N.C. Filipek, P.M. Wise, Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors alpha and beta. Endocrinology (2010). https://doi.org/10.1210/en.2010-0371
E. Vegeto, V. Benedusi, A. Maggi, Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrinol. (2008). https://doi.org/10.1016/j.yfrne.2008.04.001
D.H. Cribbs, N.C. Berchtold, V. Perreau, P.D. Coleman, J. Rogers, A.J. Tenner, C.W. Cotman, Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation (2012). https://doi.org/10.1186/1742-2094-9-179
M. Sárvári, E. Hrabovszky, I. Kalló, N. Solymosi, I. Likό, N. Berchtold, C. Cotman, Z. Liposits, Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: rat and human studies identify strikingly similar changes. J. Neuroinflammation. (2012). https://doi.org/10.1186/1742-2094-9-264
F. Yin, J. Yao, H. Sancheti, T. Feng, R.C. Melcangi, T.E. Morgan, C.E. Finch, C.J. Pike, W.J. Mack, E. Candenas, R.D. Brinton, The perimenopausal aging transition in the female rat brain: Decline in bioenergetic systems and synaptic plasticity. Neurobiol. Aging (2015). https://doi.org/10.1016/j.neurobiolaging.2015.03.013
I. Paterni, C. Granchi, J.A. Katzenellenbogen, F. Minutolo, Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids (2014). https://doi.org/10.1016/j.steroids.2014.06.012
L.-A. Haldosén, C. Zhao, K. Dahlman-Wright, Estrogen receptor beta in breast cancer. Mol. Cell. Endocrinol. (2014). https://doi.org/10.1016/j.mce.2013.08.005
B.S. McEwen, S.E. Alves, Estrogen Actions in the Central Nervous System. Endocr. Rev. (1999). https://doi.org/10.1210/edrv.20.3.0365
S.L. Petersen, E.N. Ottem, C.D. Carpenter, Direct and Indirect Regulation of Gonadotropin-Releasing Hormone Neurons by Estradiol. Biol. Reprod. (2003). https://doi.org/10.1095/biolreprod.103.019745
S.X. Simonian, D.P. Spratt, A.E. Herbison, Identification and characterization of estrogen receptor α‐containing neurons projecting to the vicinity of the gonadotropin‐releasing hormone perikarya in the rostral preoptic area of the rat. J. Comp. Neurol. (1999). https://doi.org/10.1002/(SICI)1096-9861(19990823)411:2<346::AID-CNE13>3.0.CO;2-S
B.D. Soper, R.F. Weick, Hypothalamic and extrahypothalamic mediation of pulsatile discharges of luteinizing hormone in the ovariectomized rat. Endocrinology (1980). https://doi.org/10.1210/endo-106-1-348
R. Diaz Brinton, Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology (2012). https://doi.org/10.1210/en.2012-1340
J.T. Smith, M.J. Cunningham, E.F. Rissman, D.K. Clifton, R.A. Steiner, Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology (2005). https://doi.org/10.1210/en.2005-0488
E. Hrabovszky, P.J. Shughrue, I. Merchenthaler, T. Hajszán, C.D. Carpenter, Z. Liposits, S.L. Petersen, Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology (2000). https://doi.org/10.1210/endo.141.9.7788
D. Sedlák, T.A. Wilson, W. Tjarks, H.S. Radomska, H. Wang, J. Narayana Kolla, Z.J. Leśnikowski, A. Špičáková, T. Ali, T. Ishita, L. Harinantenaina Rakotondraibe, S. Vibhute, D. Wang, P. Anzenbacher, C. Bennett, P. Barkunet, C.C. Coss, Structure–Activity Relationship of para-Carborane Selective Estrogen Receptor β Agonists. J. Med. Chem. (2021). https://doi.org/10.1021/acs.jmedchem.1c00555
C.J. Aslakson, F.R. Miller, Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 (1992)
C.N. Johnstone, Y.E. Smith, Y. Cao, A.D. Burrows, R.S.N. Cross, X. Ling, R.P. Redvers, J.P. Doherty, B.L. Eckhardt, A.L. Natoli, C.M. Restall, E. Lucas, H.B. Pearson, S. Deb, K.L. Britt, A. Rizzitelli, J. Li, J.H. Harmey, N. Pouliot, R.L. Anderson, Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis. Model. Mech. (2015). https://doi.org/10.1242/dmm.017830
L.M. Pyter, L.P. Suarez-Kelly, W.E. Carson, J. Kaur, J. Bellisario, S.R. Bever, Novel rodent model of breast cancer survival with persistent anxiety-like behavior and inflammation. Behav. Brain. Res. (2017). https://doi.org/10.1016/j.bbr.2017.05.011
D.B. Lubahn, J.S. Moyer, T.S. Golding, J.F. Couse, K.S. Korach, O. Smithies, Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA. (1993). https://doi.org/10.1073/pnas.90.23.11162
S.K.Das, J.A. Taylor, K.S. Korach, B.C. Paria, K.S. Deb, D.B. Lubahn, Estrogenic responses in estrogen receptor- deficient mice reveal a distinct estrogen signaling pathway. Proc. Natl. Acad. Sci. (1997). https://doi.org/10.1073/pnas.94.24.12786
P.J. Shughrue, D.B. Lubahn, A. Negro-Vilar, K.S. Korach, I. Merchenthaler, Responses in the brain of estrogen receptor α-disrupted mice. Proc. Natl. Acad. Sci. U.S.A. (1997). https://doi.org/10.1073/pnas.94.20.11008
K.J. Hamilton, Y. Arao, K.S. Korach, Estrogen hormone physiology: Reproductive findings from estrogen receptor mutant mice. Reprod. Biol. (2014). https://doi.org/10.1016/j.repbio.2013.12.002
P.A.S. Sheppard, E. Choleris, L.A.M. Galea, Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol. Brain (2019). https://doi.org/10.1186/s13041-019-0442-7
S.W. Mitra, E. Hoskin, J. Yudkovitz, L. Pear, H.A. Wilkinson, S. Hayashi, D.W. Pfaff, S. Ogawa, S.P. Rohrer, J.M. Schaeffer, B.S. McEwen, S.E. Alves, Immunolocalization of Estrogen Receptor β in the Mouse Brain: Comparison with Estrogen Receptor α. Endocrinology (2003). https://doi.org/10.1210/en.2002-221069
P.G. Henke, Hippocampal pathway to the amygdala and stress ulcer development. Brain. Res. Bull. (1990). https://doi.org/10.1016/0361-9230(90)90044-Z
D.S. Gross, Effect of castration and steroid replacement on immunoreactive gonadotropin-releasing hormone in the hypothalamus and preoptic area. Endocrinology (1980). https://doi.org/10.1210/endo-106-5-1442
N.J. MacLusky, A.S. Clark, F. Naftolin, P.S. Goldman-Rakic, Estrogen formation in the mammalian brain: Possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids (1987). https://doi.org/10.1016/0039-128X(87)90032-8
E. Fuente-Martin, C. Garcia-Caceres, E. Morselli, D.J. Clegg, J.A. Chowen, B. Finan, R.D. Brinton, M.H. Tschöp, Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev. Endocr. Metab. Disord. (2013). https://doi.org/10.1007/s11154-013-9263-7
A. Villa, E. Vegeto, A. Poletti, A. Maggi, Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. (2016). https://doi.org/10.1210/er.2016-1007
R.H. Straub, The complex role of estrogens in inflammation. Endocr. Rev. (2007). https://doi.org/10.1210/er.2007-0001
R. Caso, R. Silvera, R. Carrio, V. Iragavarapu-Charyulu, R.R. Gonzalez-Perez, M. Torroella-Kouri, Blood monocytes from mammary tumor-bearing mice: Early targets of tumor-induced immune suppression? Int. J. Oncol. (2010). https://doi.org/10.3892/ijo_00000740
E.J. Kovacs, T.P. Plackett, P.L. Witte, Estrogen replacement, aging, and cell-mediated immunity after injury. J. Leukoc. Biol. (2004). https://doi.org/10.1189/jlb.1103538
H.J. Son, S.H. Sohn, N. Kim, H.-N. Lee, S.M. Lee, R.H. Nam, J.H. Park, C.-H. Song, E. Shin, H.Y. Na, J.S. Kim, D.H. Lee, Y.-J. Surh, Effect of Estradiol in an Azoxymethane/Dextran Sulfate Sodium-Treated Mouse Model of Colorectal Cancer: Implication for Sex Difference in Colorectal Cancer Development. Cancer Res. Treat. (2019). https://doi.org/10.4143/crt.2018.060
E.T. Fantozzi, A.C. Breithaupt-Faloppa, F.Y. Ricardo-da-Silva, S. Rodrigues-Garbin, D.C. Romero, A. da Silva Rodrigues, Y. Riffo-Vasquez, W. Taveres-de-Lima, Estradiol mediates the long-lasting lung inflammation induced by intestinal ischemia and reperfusion. J. Surg. Res. (2018). https://doi.org/10.1016/j.jss.2017.07.038
M. Yang, J. Kim, J.-S. Kim, S.-H. Kim, J.-C. Kim, M.-J. Kang, U. Jung, T. Shin, H. Wang, C. Moon, Hippocampal dysfunctions in tumor-bearing mice. Brain Behav. Immun. (2014). https://doi.org/10.1016/j.bbi.2013.10.022
A.J. Bruce-Keller, J.L. Keeling, J.N. Keller, F.F. Huang, S. Camondola, M.P. Mattson, Antiinflammatory Effects of Estrogen on Microglial Activation. Endocrinology (2000). https://doi.org/10.1210/endo.141.10.7693
Y. Xu, H. Sheng, Z. Tang, J. Lu, X. Ni, Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav. Brain Res. (2015). https://doi.org/10.1016/j.bbr.2015.04.017
Z. Tian, J. Fan, Y. Zhao, S. Bi, L. Si, Q. Liu, Estrogen receptor beta treats Alzheimer’s disease. Neural Regen. Res. (2013). https://doi.org/10.3969/j.issn.1673-5374.2013.05.005
K.G. Vargas, J. Milic, A. Zaciragic, K.-X. Wen, L. Jaspers, J. Nano, K. Dhana, W.M. Bramer, B. Kraja, E. van Beeck, M.A. Ikram, T. Muka, O.H. Franco, The functions of estrogen receptor beta in the female brain: a systematic review. Maturitas (2016). https://doi.org/10.1016/j.maturitas.2016.05.014
V.L. Nordell, M.M. Scarborough, A.K. Buchanan, F. Sohrabji, Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol. Aging (2003). https://doi.org/10.1016/S0197-4580(02)00193-8
J.C. Santos, S.R. Bever, G. Pereira-da-Silva, L.M. Pyter, Tumor resection ameliorates tumor-induced suppression of neuroinflammatory and behavioral responses to an immune challenge in a cancer survivor model. Sci. Rep. (2019). https://doi.org/10.1038/s41598-018-37334-8
E. Ogura, K. Kageyama, K. Hanada, J. Kasckow, T. Suda, Effects of estradiol on regulation of corticotropin-releasing factor gene and interleukin-6 production via estrogen receptor type β in hypothalamic 4B cells. Peptides (2008). https://doi.org/10.1016/j.peptides.2007.11.007
Acknowledgements
The authors thank Lindsay Strehle, Valerie Burch, Ann Thomas, and Aliza Khuro for their skilled technical assistance, as well as Megan Fleming and Dr. Stacey Meeker for animal husbandry and veterinary support, respectively. We also acknowledge Drs. Christopher Coss and Chad Bennet of The Ohio State University, Comprehensive Cancer Center, Drug Development Institute for their technical help using and quantifying the novel compound.
Author contributions
K.L.G.R. and L.M.P. contributed to the conceptual design of the study. K.L.G.R. and C.V.G. were responsible for data collection and analysis. All authors contributed to data interpretation, paper preparation, and approved the final version of the paper for submission.
Funding
This work was supported by The Ohio State University Comprehensive Cancer Center’s Drug Development Institute (institutional grant, 2019), funds from The Ohio State College of Medicine, and a NIH/NCI fellowship (K.L.G.R.) [T32CA009338].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The data described here are original and have not been previously published. All procedures and experiments were approved by The Ohio state University Institutional Animal Care and Use Committee (IACUC, 2014A00000093, approved 08/04/2020) and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Grant, C.V., Russart, K.L.G. & Pyter, L.M. A novel targeted approach to delineate a role for estrogen receptor-β in ameliorating murine mammary tumor-associated neuroinflammation. Endocrine 75, 949–958 (2022). https://doi.org/10.1007/s12020-021-02931-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02931-7