Abstract
Purpose
To estimate the prevalence of USP8, USP48, and BRAF mutations in patients with Cushing’s disease (CD) from the Indian subcontinent, and determine their genotype-phenotype correlation.
Methods
We prospectively recruited 46 patients with CD who underwent surgery between September 2015 and July 2019 at our institute. Fresh frozen tumour tissue was obtained in all patients. Using Sanger sequencing, the presence of somatic USP8 mutations was documented and the frequency of USP48 and BRAF mutations in USP8 wild-type corticotroph adenomas was determined. Clinical, hormonal, and surgical data were then compared between USP8-, USP48- and BRAF-variant carriers and patients with wild-type tumours.
Results
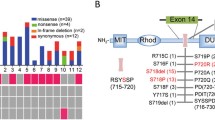
Signature USP8 mutations were detected in 17 (37%) patients. Of the 29 USP8 wild-type adenomas, 4 (13.8%) harboured USP48 mutations, one of them being a splice-site mutation that has previously not been described. BRAF mutations were not found in any of the 29 patients. Corticotroph adenomas with USP8 mutations had a higher incidence of Crooke’s hyaline change than wild-type tumours (70.6 vs. 37.9%, p = 0.032). Adenomas with USP48 mutations had a higher rate of cavernous sinus invasion than their wild-type counterparts (50 vs. 4%, p = 0.042). No other significant phenotypic difference could be established between mutant and wild-type tumours.
Conclusions
The prevalence of USP8 mutations in our series of patients with CD was 37%. The prevalence of USP48 mutations in USP8 wild-type adenomas was 13.8%, including a novel splice-site mutation. BRAF mutations were not found in any USP8 wild-type tumour. USP8-mutants showed significantly more Crooke’s hyaline change and USP48-mutants were more likely to demonstrate cavernous sinus invasion.


Similar content being viewed by others
Data availability
On request
References
M. Reincke, S. Sbiera, A. Hayakawa, M. Theodoropoulou, A. Osswald, F. Beuschlein, T. Meitinger, E. Mizuno-Yamasaki, K. Kawaguchi, Y. Saeki, K. Tanaka, T. Wieland, E. Graf, W. Saeger, C.L. Ronchi, B. Allolio, M. Buchfelder, T.M. Strom, M. Fassnacht, M. Komada, Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 47, 31–38 (2014). https://doi.org/10.1038/ng.3166
L.G. Perez-Rivas, M. Theodoropoulou, F. Ferraù, C. Nusser, K. Kawaguchi, C.A. Stratakis, F. Rueda Faucz, L.E. Wildemberg, G. Assié, R. Beschorner, C. Dimopoulou, M. Buchfelder, V. Popovic, C.M. Berr, M. Tóth, A.I. Ardisasmita, J. Honegger, J. Bertherat, M.R. Gadelha, F. Beuschlein, G. Stalla, M. Komada, M. Korbonits, M. Reincke, The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J. Clin. Endocrinol. Metab. 100, E997–1004 (2015). https://doi.org/10.1210/jc.2015-1453
Z.-Y. Ma, Z.-J. Song, J.-H. Chen, Y.-F. Wang, S.-Q. Li, L.-F. Zhou, Y. Mao, Y.-M. Li, R.-G. Hu, Z.-Y. Zhang, H.-Y. Ye, M. Shen, X.-F. Shou, Z.-Q. Li, H. Peng, Q.-Z. Wang, D.-Z. Zhou, X.-L. Qin, J. Ji, J. Zheng, H. Chen, Y. Wang, D.-Y. Geng, W.-J. Tang, C.-W. Fu, Z.-F. Shi, Y.-C. Zhang, Z. Ye, W.-Q. He, Q.-L. Zhang, Q.-S. Tang, R. Xie, J.-W. Shen, Z.-J. Wen, J. Zhou, T. Wang, S. Huang, H.-J. Qiu, N.-D. Qiao, Y. Zhang, L. Pan, W.-M. Bao, Y.-C. Liu, C.-X. Huang, Y.-Y. Shi, Y. Zhao, Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 25, 306–317 (2015). https://doi.org/10.1038/cr.2015.20
C. Ballmann, A. Thiel, H.E. Korah, A.-C. Reis, W. Saeger, S. Stepanow, K. Köhrer, G. Reifenberger, C.B. Knobbe-Thomsen, U.J. Knappe, U.I. Scholl, USP8 mutations in Pituitary Cushing adenomas—Targeted analysis by next-generation sequencing. J. Endocr. Soc. 2, 266–278 (2018). https://doi.org/10.1210/js.2017-00364
M. Losa, P. Mortini, A. Pagnano, M. Detomas, M.F. Cassarino, F. Pecori Giraldi, Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine 63, 240–246 (2018). https://doi.org/10.1007/s12020-018-1776-0
K. Hayashi, N. Inoshita, K. Kawaguchi, A. Ibrahim Ardisasmita, H. Suzuki, N. Fukuhara, M. Okada, H. Nishioka, Y. Takeuchi, M. Komada, A. Takeshita, S. Yamada, The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur. J. Endocrinol. 174, 213–226 (2016). https://doi.org/10.1530/EJE-15-0689
I. Weigand, L. Knobloch, J. Flitsch, W. Saeger, C.M. Monoranu, K. Höfner, S. Herterich, R. Rotermund, C.L. Ronchi, M. Buchfelder, M. Glatzel, C. Hagel, M. Fassnacht, T. Deutschbein, S. Sbiera, Impact of USP8 gene mutations on protein deregulation in Cushing disease. J. Clin. Endocrinol. Metab. 104, 2535–2546 (2019). https://doi.org/10.1210/jc.2018-02564
M. Riebold, C. Kozany, L. Freiburger, M. Sattler, M. Buchfelder, F. Hausch, G.K. Stalla, M. Paez-Pereda, A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease. Nat. Med. 21, 276–280 (2015). https://doi.org/10.1038/nm.3776
J. Chen, X. Jian, S. Deng, Z. Ma, X. Shou, Y. Shen, Q. Zhang, Z. Song, Z. Li, H. Peng, C. Peng, M. Chen, C. Luo, D. Zhao, Z. Ye, M. Shen, Y. Zhang, J. Zhou, A. Fahira, Y. Wang, S. Li, Z. Zhang, H. Ye, Y. Li, J. Shen, H. Chen, F. Tang, Z. Yao, Z. Shi, C. Chen, L. Xie, Y. Wang, C. Fu, Y. Mao, L. Zhou, D. Gao, H. Yan, Y. Zhao, C. Huang, Y. Shi, Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat. Commun. 9, 3171 (2018). https://doi.org/10.1038/s41467-018-05275-5
S. Sbiera, L.G. Perez-Rivas, L. Taranets, I. Weigand, J. Flitsch, E. Graf, C.-M. Monoranu, W. Saeger, C. Hagel, J. Honegger, G. Assie, A.R. Hermus, G.K. Stalla, S. Herterich, C.L. Ronchi, T. Deutschbein, M. Reincke, T.M. Strom, N. Popov, M. Theodoropoulou, M. Fassnacht, Driver mutations in USP8 wild-type Cushing’s disease. Neuro-Oncol. 21, 1273–1283 (2019). https://doi.org/10.1093/neuonc/noz109
S. Sarkar, S. Rajaratnam, G. Chacko, S. Mani, A.S. Hesargatta, A.G. Chacko, Pure endoscopic transsphenoidal surgery for functional pituitary adenomas: Outcomes with Cushing’s disease. Acta Neurochir. (Wien.) 158, 77–86 (2016). https://doi.org/10.1007/s00701-015-2638-7
M. Ferchichi, R. Jouini, I. Ayari, W. Koubaa, A. Chadli-Debbiche, E. BenBrahim, KRAS, NRAS and BRAF analysis of ampullary adenocarcinoma classified using CK7, CK20, MUC1 and MUC2. J. Gastrointest. Oncol. 9, 820–827 (2018). https://doi.org/10.21037/jgo.2018.05.03
F.R. Faucz, A. Tirosh, C. Tatsi, A. Berthon, L.C. Hernández-Ramírez, N. Settas, A. Angelousi, R. Correa, G.Z. Papadakis, P. Chittiboina, M. Quezado, N. Pankratz, J. Lane, A. Dimopoulos, J.L. Mills, M. Lodish, C.A. Stratakis, Somatic USP8 gene mutations are a common cause of pediatric Cushing disease. J. Clin. Endocrinol. Metab. 102, 2836–2843 (2017). https://doi.org/10.1210/jc.2017-00161
A. Albani, L.G. Pérez-Rivas, C. Dimopoulou, S. Zopp, P. Colón-Bolea, S. Roeber, J. Honegger, J. Flitsch, W. Rachinger, M. Buchfelder, G.K. Stalla, J. Herms, M. Reincke, M. Theodoropoulou, The USP8 mutational status may predict long-term remission in patients with Cushing’s disease. Clin. Endocrinol. (Oxf.) 89, 454–458 (2018). https://doi.org/10.1111/cen.13802
S. Sbiera, M. Kunz, I. Weigand, T. Deutschbein, T. Dandekar, M. Fassnacht, The new genetic landscape of Cushing’s disease: Deubiquitinases in the spotlight. Cancers 11, 1761 (2019). https://doi.org/10.3390/cancers11111761
L.G. Pérez-Rivas, M. Theodoropoulou, T.H. Puar, J. Fazel, M.R. Stieg, F. Ferraù, G. Assié, M.R. Gadelha, T. Deutschbein, M.C. Fragoso, B. Kusters, W. Saeger, J. Honegger, M. Buchfelder, M. Korbonits, J. Bertherat, G.K. Stalla, A.R. Hermus, F. Beuschlein, M. Reincke, Somatic USP8 mutations are frequent events in corticotroph tumor progression causing Nelson’s tumor. Eur. J. Endocrinol. 178, 57–63 (2018). https://doi.org/10.1530/EJE-17-0634
L.G. Libuit, A.S. Karageorgiadis, N. Sinaii, N.M. Nguyen May, M.F. Keil, M.B. Lodish, C.A. Stratakis, A gender-dependent analysis of Cushing’s disease in childhood: Pre- and postoperative follow-up. Clin. Endocrinol. (Oxf.) 83, 72–77 (2015). https://doi.org/10.1111/cen.12644
S.S. Chaidarun, B. Swearingen, J.M. Alexander, Differential expression of estrogen receptor-beta (ER beta) in human pituitary tumors: functional interactions with ER alpha and a tumor-specific splice variant. J. Clin. Endocrinol. Metab. 83, 3308–3315 (1998). https://doi.org/10.1210/jcem.83.9.5128
S. Oomizu, J. Honda, S. Takeuchi, T. Kakeya, T. Masui, S. Takahashi, Transforming growth factor-alpha stimulates proliferation of mammotrophs and corticotrophs in the mouse pituitary. J. Endocrinol. 165, 493–501 (2000). https://doi.org/10.1677/joe.0.1650493
E.H. Oldfield, A.O.J. Vortmeyer, Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. Neurosurg. 104, 7–19 (2006). https://doi.org/10.3171/jns.2006.104.1.7
Acknowledgements
We would like to sincerely thank L Jeyaseelan and Malavika Babu from the Department of Biostatistics for their help with the statistical analysis of the data. The findings of this study were presented at the annual conference of the Indian Society of Neuro-Oncology, 2021.
Author contributions
APA, AGC, RP, GC, and HSA contributed to conception and design of the study. AGC operated on the patients in this study. GC supervised the histopathological examination of all tumours. DLB and RP did DNA sequencing of the tumor samples. HSA, SR, NT, and NK were responsible for diagnosis of the patients with Cushing’s disease and their medical management. APA collected patient information, performed statistical analysis and interpretation of data, and wrote the first draft of the paper. All authors critically reviewed the paper. All authors read and approved the final version of the paper.
Funding
The study was funded by an intramural research grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study was approved by the ethics committee of our Institutional Review Board (IRB Min No: 9628, dated 01.09.2015). Informed consent was obtained from all adult patients. In the case of minors, assent was obtained from patients along with the informed consent of their parents/guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Abraham, A.P., Pai, R., Beno, D.L. et al. USP8, USP48, and BRAF mutations differ in their genotype-phenotype correlation in Asian Indian patients with Cushing’s disease. Endocrine 75, 549–559 (2022). https://doi.org/10.1007/s12020-021-02903-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02903-x