Abstract
Background
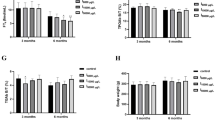
Thyroid damage occurs during experimental iodine-deficient goiter and involution with iodine supplementation. This study investigated the dynamic microRNAs (miRNAs) expression profiles in iodine-deficient thyroids during adequate and excessive iodine supplementation.
Methods
Twenty-four female Wistar rats were randomly divided into control, low-iodine (LI), LI-1I, and LI-2I groups. The LI-1I and LI-2I groups were fed a LI diet for 12 weeks, followed by a onefold (adequate) or twofold (excessive) physiological dose of iodine for 4 weeks to induce involution. The miRNA expression profiles were evaluated and the potential functions of the differentially expressed miRNAs identified were explored.
Results
In the LI group, 20 miRNAs were downregulated and 8 were upregulated. After involution, 21 miRNAs recovered to the control group levels in the LI-1I group, which was more than the 17 that recovered in the LI-2I group. In addition, 8 new differentially expressed miRNAs were identified in the LI-1I group, which was less than the 13 found in the LI-2I group. Bioinformatics analyses indicated that all differentially expressed miRNAs were involved in different processes and pathways, such as autoimmune thyroid disease and the Ras signaling pathway.
Conclusion
Differentially expressed miRNAs are involved in iodine-deficient goiter formation and involution. Supplementation with adequate, not excessive, iodine may be more beneficial to restore homeostasis.






Similar content being viewed by others
References
A. Carle, A. Krejbjerg, P. Laurberg, Epidemiology of nodular goitre. Influence of iodine intake. Best practice & research. Best Pract. Res. Clin. Endocrinol. Metab. 28(4), 465–479 (2014). https://doi.org/10.1016/j.beem.2014.01.001
M. Chaudhary, N. Baisakhiya, G. Singh, Clinicopathological and radiological study of thyroid swelling. Indian J. Otolaryngol. Head Neck Surg. 71(Suppl 1), 893–904 (2019). https://doi.org/10.1007/s12070-019-01616-y
K. Markou, N. Georgopoulos, V. Kyriazopoulou, A.G. Vagenakis, Iodine-induced hypothyroidism. Thyroid 11(5), 501–510 (2001). https://doi.org/10.1089/105072501300176462
A.M. Leung, L.E. Braverman, Iodine-induced thyroid dysfunction. Curr. Opin. Endocrinol. Diabetes Obes. 19(5), 414–419 (2012). https://doi.org/10.1097/MED.0b013e3283565bb2
W. Teng, Z. Shan, X. Teng, H. Guan, Y. Li, D. Teng, Y. Jin, X. Yu, C. Fan, W. Chong, F. Yang, H. Dai, Y. Yu, J. Li, Y. Chen, D. Zhao, X. Shi, F. Hu, J. Mao, X. Gu, R. Yang, Y. Tong, W. Wang, T. Gao, C. Li, Effect of iodine intake on thyroid diseases in China. N. Engl. J. Med. 354(26), 2783–2793 (2006). https://doi.org/10.1056/NEJMoa054022
P. Laurberg, T. Jorgensen, H. Perrild, L. Ovesen, N. Knudsen, I.B. Pedersen, L.B. Rasmussen, A. Carle, P. Vejbjerg, The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur. J. Endocrinol. 155(2), 219–228 (2006). https://doi.org/10.1530/eje.1.02210
S. Poncin, A.C. Gérard, M. Boucquey, M. Senou, P.B. Calderon, B. Knoops, B. Lengelé, M.C. Many, I.M. Colin, Oxidative stress in the thyroid gland: from harmlessness to hazard depending on the iodine content. Endocrinology 149(1), 424–433 (2008). https://doi.org/10.1210/en.2007-0951
J.F. Mutaku, J.F. Poma, M.C. Many, J.F. Denef, M.F. van Den Hove, Cell necrosis and apoptosis are differentially regulated during goitre development and iodine-induced involution. J. Endocrinol. 172(2), 375–386 (2002). https://doi.org/10.1677/joe.0.1720375
M.C. Many, J.F. Denef, Iodine and goiter involution. Thyroidology 4(1), 23–26 (1992)
M.C. Many, S. Maniratunga, I. Varis, M. Dardenne, H.A. Drexhage, J.F. Denef, Two-step development of Hashimoto-like thyroiditis in genetically autoimmune prone non-obese diabetic mice: effects of iodine-induced cell necrosis. J. Endocrinol. 147(2), 311–320 (1995). https://doi.org/10.1677/joe.0.1470311
W. Dong, H. Zhang, P. Zhang, X. Li, L. He, Z. Wang, Y. Liu, The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med. Sci. Monit. 19, 49–53 (2013). https://doi.org/10.12659/msm.883736
M. Blomberg, U. Feldt-Rasmussen, K.K. Andersen, S.K. Kjaer, Thyroid cancer in Denmark 1943-2008, before and after iodine supplementation. Int. J. Cancer 131(10), 2360–2366 (2012). https://doi.org/10.1002/ijc.27497
G. Wan, R. Mathur, X. Hu, X. Zhang, X. Lu, miRNA response to DNA damage. Trends Biochem. Sci. 36(9), 478–484 (2011). https://doi.org/10.1016/j.tibs.2011.06.002
D.P. Bartel, MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2), 281–297 (2004). https://doi.org/10.1016/s0092-8674(04)00045-5
H. Otsu, M. Watanabe, N. Inoue, R. Masutani, Y. Iwatani, Intraindividual variation of microRNA expression levels in plasma and peripheral blood mononuclear cells and the associations of these levels with the pathogenesis of autoimmune thyroid diseases. Clin. Chem. Lab. Med. 55(5), 626–635 (2017). https://doi.org/10.1515/cclm-2016-0449
M. Sun, S. Fang, W. Li, C. Li, L. Wang, F. Wang, Y. Wang, Associations of miR-146a and miR-146b expression and clinical characteristics in papillary thyroid carcinoma. Cancer Biomark. 15(1), 33–40 (2015). https://doi.org/10.3233/cbm-140431
M.E. Graham, R.D. Hart, S. Douglas, F.M. Makki, D. Pinto, A.L. Butler, M. Bullock, M.H. Rigby, J.R. Trites, S.M. Taylor, R. Singh, Serum microRNA profiling to distinguish papillary thyroid cancer from benign thyroid masses. J. Otolaryngol. Head Neck Surg. 44, 33 (2015). https://doi.org/10.1186/s40463-015-0083-5
A. Sondermann, F.M. Andreghetto, A.C. Moulatlet, E. da Silva Victor, M.G. de Castro, F.D. Nunes, L.G. Brandão, P. Severino, MiR-9 and miR-21 as prognostic biomarkers for recurrence in papillary thyroid cancer. Clin. Exp. Metastasis 32(6), 521–530 (2015). https://doi.org/10.1007/s10585-015-9724-3
J. Yu, Z. Shan, W. Chong, J. Mao, Y. Geng, C. Zhang, Q. Xing, W. Wang, N. Li, C. Fan, H. Wang, H. Zhang, W. Teng, Vitamin E ameliorates iodine-induced cytotoxicity in thyroid. J. Endocrinol. 209(3), 299–306 (2011). https://doi.org/10.1530/joe-11-0030
L. Zheng, C. Zhuang, X. Wang, L. Ming, Serum miR-146a, miR-155, and miR-210 as potential markers of Graves’ disease. J. Clin. Lab. Anal. 32(2) (2018). https://doi.org/10.1002/jcla.22266
Q. Qin, X. Wang, N. Yan, R.H. Song, T.T. Cai, W. Zhang, L.J. Guan, F.S. Muhali, J.A. Zhang, Aberrant expression of miRNA and mRNAs in lesioned tissues of Graves’ disease. Cell. Physiol. Biochem. 35(5), 1934–1942 (2015). https://doi.org/10.1159/000374002
Z. Sun, L. Yi, H. Tao, J. Huang, Z. Jin, Y. Xiao, C. Feng, J. Sun, Enhancement of soluble CD28 levels in the serum of Graves’ disease. Cent. Eur. J. Immunol. 39(2), 216–222 (2014). https://doi.org/10.5114/ceji.2014.43726
H. Yamada, M. Itoh, I. Hiratsuka, S. Hashimoto, Circulating microRNAs in autoimmune thyroid diseases. Clin. Endocrinol. 81(2), 276–281 (2014). https://doi.org/10.1111/cen.12432
R.T. Schaller, J.K. Stevenson, Development of carcinoma of the thyroid in iodine-deficient mice. Cancer 19(8), 1063–1080 (1966). 10.1002/1097-0142(196608)19:8<1063::aid-cncr2820190804>3.0.co;2-a
S.D. Mitro, L.S. Rozek, P. Vatanasapt, K. Suwanrungruang, I. Chitapanarux, S. Srisukho, H. Sriplung, R. Meza, Iodine deficiency and thyroid cancer trends in three regions of Thailand, 1990-2009. Cancer Epidemiol. 43, 92–99 (2016). https://doi.org/10.1016/j.canep.2016.07.002
B. Zha, X. Huang, J. Lin, J. Liu, Y. Hou, G. Wu, Distribution of lymphocyte subpopulations in thyroid glands of human autoimmune thyroid disease. J. Clin. Lab. Anal. 28(3), 249–254 (2014). https://doi.org/10.1002/jcla.21674
M. Bastemir, R. Emral, G. Erdogan, S. Gullu, High prevalence of thyroid dysfunction and autoimmune thyroiditis in adolescents after elimination of iodine deficiency in the Eastern Black Sea Region of Turkey. Thyroid 16(12), 1265–1271 (2006). https://doi.org/10.1089/thy.2006.16.1265
Y. Luo, A. Kawashima, Y. Ishido, A. Yoshihara, K. Oda, N. Hiroi, T. Ito, N. Ishii, K. Suzuki, Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int. J. Mol. Sci. 15(7), 12895–12912 (2014). https://doi.org/10.3390/ijms150712895
G.H. McIntosh, G.B. Jones, D.A. Howard, G.B. Belling, B.J. Potter, B.S. Hetzel, Low-iodine diet for producing iodine deficiency in rats. Aust. J. Biol. Sci. 33(2), 205–211 (1980). https://doi.org/10.1071/bi9800205
W. Chong, W. Chen, W. Teng, Y. Jin, X. Zhang, L. Xi, N. Zhang, N. Man, Y. Tong, Y. Yu, C. Fan, H. Guan, Y. Li, Establish and evaluation ofiodine-deficient animal model. J. Chin. Med. Univ. 34(2), 111–113 (2005). (In Chinese)
Acknowledgements
We thank Medjaden Bioscience Limited for English editing.
Funding
This work was funded by W.C. (Grant Number 81372970) and J.M. (Grant Number LFWK201702 and LR2019075).
Author information
Authors and Affiliations
Contributions
Data analysis and writing—original draft preparation: J.Z. and J.M.; methodology: J.Y. and W.C.; project supervision: Z.S. and W.T.; data analysis: J.Z, C.L and J.M.; funding acquisition: W.C. and J.M.; and project administration and writing—review and editing: J.M.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All procedures performed in studies involving rats were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, J., Yu, J., Shan, Z. et al. MicroRNA expression profiles of the thyroid after goiter formation and involution in rats under different iodine regimens. Endocrine 73, 598–608 (2021). https://doi.org/10.1007/s12020-021-02679-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02679-0