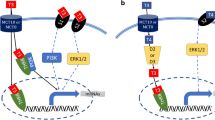
Abstract
Background and aim
Skeletal muscle (SM) has been shown as a target of thyroid hormones (THs). However, the status of TH signaling in aged SM remains unclear. This study aimed to explore the mechanism of TH signaling in SM of aging mice.
Methods
Thirty C57BL/6J male mice were divided into 6-, 15- and 22-month (6, 15 and 22M) groups according to different age. Physical parameters were evaluated by analytical balance, grip strength test and histological analysis. Thyroid function was detected by enzyme-linked immunosorbent assay. TH signaling was compared among the three groups by real-time PCR and western blotting analysis.
Results
p16, p21, and p53 mRNA levels in SM increased in age-dependent manner. The muscle weight and strength decreased in 22M group compared to 6 and 15M groups. Concentrations of thyroid hormones, including free triiodothyronine (FT3), free thyroxine (FT4) and thyroid-stimulating hormone (TSH) in 22 M mice were not shown significant difference compared to 6M or 15M mice, although FT3 showed slightly decrease and TSH appeared a mild increase accompanying with age. mRNA levels of TH transporters, including MCT8 and MCT10, as well as iodothyronine deiodinase type 2 (DIO2) and type 3 (DIO3), were higher in 22M, while TH receptor α (TRα) mRNA and protein expression was lower in 22M, compared to the other groups. Type-I myosin heavy chain (MyHC I), MyHC IIx, and MyHC IIa were upregulated and Type-IIb MyHC (MyHC IIb) was downregulated in SM with advancing age.
Conclusions
TH signaling in SM changes with aging.







Similar content being viewed by others
References
J.P. da Costa, R. Vitorino, G.M. Silva, C. Vogel, A.C. Duarte, T. Rocha-Santos, A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 29, 90–112 (2016). https://doi.org/10.1016/j.arr.2016.06.005
A.J. Cruz-Jentoft, G. Bahat, J. Bauer, Y. Boirie, O. Bruyere, T. Cederholm, C. Cooper, F. Landi, Y. Rolland, A.A. Sayer, S.M. Schneider, C.C. Sieber, E. Topinkova, M. Vandewoude, M. Visser, M. Zamboni, Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1), 16–31 (2019). https://doi.org/10.1093/ageing/afy169
M. Locquet, C. Beaudart, J. Petermans, J.Y. Reginster, O. Bruyere, EWGSOP2 versus EWGSOP1: impact on the prevalence of sarcopenia and its major health consequences. J. Am. Med. Dir. Assoc. 20(3), 384–385 (2019). https://doi.org/10.1016/j.jamda.2018.11.027
Y. Rolland, S. Czerwinski, G. Abellan Van Kan, J.E. Morley, M. Cesari, G. Onder, J. Woo, R. Baumgartner, F. Pillard, Y. Boirie, W.M. Chumlea, B. Vellas, Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 12(7), 433–450 (2008). https://doi.org/10.1007/bf02982704
W.R. Frontera, J. Ochala, Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int. 96(3), 183–195 (2015). https://doi.org/10.1007/s00223-014-9915-y
S. Schiaffino, C. Reggiani, Fiber types in mammalian skeletal muscles. Physiol. Rev. 91(4), 1447–1531 (2011). https://doi.org/10.1152/physrev.00031.2010
F.F. Bloise, A. Cordeiro, T.M. Ortiga-Carvalho, Role of thyroid hormone in skeletal muscle physiology. J. Endocrinol. 236(1), R57–r68 (2018). https://doi.org/10.1530/joe-16-0611
W.S. Simonides, C. van Hardeveld, Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 18(2), 205–216 (2008). https://doi.org/10.1089/thy.2007.0256
G.A. Brent, Commentary on: “American Thyroid Association Guide to investigating thyroid hormone economy and action in rodent and cell models,” Bianco et al. Thyroid 24(1), 1–2 (2014). https://doi.org/10.1089/thy.2013.0679
A.C. Bianco, A. Dumitrescu, B. Gereben, M.O. Ribeiro, T.L. Fonseca, G.W. Fernandes, B. Bocco, Paradigms of dynamic control of thyroid hormone signaling. Endocr. Rev. 40(4), 1000–1047 (2019). https://doi.org/10.1210/er.2018-00275
T. Kadoguchi, K. Shimada, T. Miyazaki, K. Kitamura, M. Kunimoto, T. Aikawa, Y. Sugita, S. Ouchi, T. Shiozawa, M. Yokoyama-Nishitani, K. Fukao, K. Miyosawa, K. Isoda, H. Daida, Promotion of oxidative stress is associated with mitochondrial dysfunction and muscle atrophy in aging mice. Geriatr. Gerontol. Int. 20(1), 78–84 (2020). https://doi.org/10.1111/ggi.13818
D. Zhang, Y. Li, S. Liu, Y.C. Wang, F. Guo, Q. Zhai, J. Jiang, H. Ying, microRNA and thyroid hormone signaling in cardiac and skeletal muscle. Cell Biosci. 7, 14 (2017). https://doi.org/10.1186/s13578-017-0141-y
Y. Sheng, D. Ma, Q. Zhou, L. Wang, M. Sun, S. Wang, H. Qi, J. Liu, G. Ding, Y. Duan, Association of thyroid function with sarcopenia in elderly Chinese euthyroid subjects. Aging Clin. Exp. Res. 31(8), 1113–1120 (2019). https://doi.org/10.1007/s40520-018-1057-z
Y. Duan, C. Liu, S. Feng, X. Wang, W. Tang, X. Mao, S. Xu, Y. Feng, H. Shen, R. Yu, R. Bu, J. Chen, W. Li, Z. Shi, X. Hu, Epidemiologic study of hypothyroidism in Jiangsu province. Chin. J. Endocrinol. Metab. 24(3), 275–277 (2008)
T.M. Ortiga-Carvalho, M.I. Chiamolera, C.C. Pazos-Moura, F.E. Wondisford, Hypothalamus-pituitary-thyroid axis. Compr. Physiol. 6(3), 1387–1428 (2016). https://doi.org/10.1002/cphy.c150027
E.C. Friesema, J. Jansen, J.W. Jachtenberg, W.E. Visser, M.H. Kester, T.J. Visser, Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol. Endocrinol. 22(6), 1357–1369 (2008). https://doi.org/10.1210/me.2007-0112
L. Mebis, D. Paletta, Y. Debaveye, B. Ellger, L. Langouche, A. D’Hoore, V.M. Darras, T.J. Visser, G. Van den Berghe, Expression of thyroid hormone transporters during critical illness. Eur. J. Endocrinol. 161(2), 243–250 (2009). https://doi.org/10.1530/eje-09-0290
C. Di Cosmo, X.H. Liao, A.M. Dumitrescu, N.J. Philp, R.E. Weiss, S. Refetoff, Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J. Clin. Investig. 120(9), 3377–3388 (2010). https://doi.org/10.1172/JCI42113
D. Salvatore, W.S. Simonides, M. Dentice, A.M. Zavacki, P.R. Larsen, Thyroid hormones and skeletal muscle–new insights and potential implications. Nat. Rev. Endocrinol. 10(4), 206–214 (2014). https://doi.org/10.1038/nrendo.2013.238
R. Ambrosio, M.A. De Stefano, D. Di Girolamo, D. Salvatore, Thyroid hormone signaling and deiodinase actions in muscle stem/progenitor cells. Mol. Cell. Endocrinol. 459, 79–83 (2017). https://doi.org/10.1016/j.mce.2017.06.014
A. Boelen, A.H. van der Spek, F. Bloise, E.M. de Vries, O.V. Surovtseva, M. van Beeren, M.T. Ackermans, J. Kwakkel, E. Fliers, Tissue thyroid hormone metabolism is differentially regulated during illness in mice. J. Endocrinol. 233(1), 25–36 (2017). https://doi.org/10.1530/joe-16-0483
G.A. Brent, Mechanisms of thyroid hormone action. J. Clin. Investig. 122(9), 3035–3043 (2012). https://doi.org/10.1172/jci60047
S.Y. Cheng, J.L. Leonard, P.J. Davis, Molecular aspects of thyroid hormone actions. Endocr. Rev. 31(2), 139–170 (2010). https://doi.org/10.1210/er.2009-0007
J.H. Oppenheimer, H.L. Schwartz, M.I. Surks, Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology 95(3), 897–903 (1974). https://doi.org/10.1210/endo-95-3-897
J.E. Silva, T.E. Dick, P.R. Larsen, The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3’-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology 103(4), 1196–1207 (1978). https://doi.org/10.1210/endo-103-4-1196
A. Tylki-Szymanska, R. Acuna-Hidalgo, M. Krajewska-Walasek, A. Lecka-Ambroziak, M. Steehouwer, C. Gilissen, H.G. Brunner, A. Jurecka, A. Rozdzynska-Swiatkowska, A. Hoischen, K.H. Chrzanowska, Thyroid hormone resistance syndrome due to mutations in the thyroid hormone receptor alpha gene (THRA). J. Med. Genet. 52(5), 312–316 (2015). https://doi.org/10.1136/jmedgenet-2014-102936
F. Yu, S. Gothe, L. Wikstrom, D. Forrest, B. Vennstrom, L. Larsson, Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278(6), R1545–R1554 (2000). https://doi.org/10.1152/ajpregu.2000.278.6.R1545
S. Schiaffino, A.C. Rossi, V. Smerdu, L.A. Leinwand, C. Reggiani, Developmental myosins: expression patterns and functional significance. Skelet. Muscle 5, 22 (2015). https://doi.org/10.1186/s13395-015-0046-6
Acknowledgements
The authors appreciate all of individuals for their assistance in this study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81670724) to Y.D.
Author contributions
Y.D.: experiments design and revise. W.X.: performing the experiments. L.W. and Y.S.: data analysis and interpretation. M.S., S.L. and J.Y.: critical review of this paper. X.W. and G.D.: editing this paper. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Animal studies were in agreement with series of ethical standards, including the institutional and/or national research committee, 1964 Helsinki declaration and its later amendments, and the National and Institutional Guidelines for Animal Welfare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Sheng, Y., Xu, W. et al. Mechanism of thyroid hormone signaling in skeletal muscle of aging mice. Endocrine 72, 132–139 (2021). https://doi.org/10.1007/s12020-020-02428-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02428-9