Abstract
Objective
To investigate whether gonadotropin releasing hormone analogue (GnRHa) combined with recombinant human growth hormone (rhGH) can improve the adult height (AHt) of children with short stature and normal pubertal onset.
Methods
In this retrospective study, GnRHa/rhGH treatment was given to children with normal pubertal onset and short stature. Patients were followed up to measure their AHt. The primary outcomes were the disparity between AHt standard deviation score (AHt SDS) and pre-treatment height standard deviation score (Ht SDS) and the disparity between AHt and target height (THt).
Results
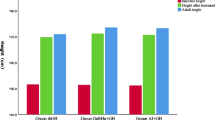
A total of 94 patients were included. Forty-nine boys were treated with GnRHa/rhGH for 24.84 ± 13.01 months, and 45 girls were treated for 23.89 ± 10.43 months. (2) Before treatment, the Ht SDS of boys and girls was −1.82 ± 1.30 and −1.10 ± 1.61, respectively, and the target height was 168.98 ± 3.51 cm and 157.90 ± 3.25 cm, respectively. (3) After treatment, for boys, the AHt SDS increased by 1.37 ± 1.28 (p = 0.000) and the disparity between AHt and THt was 0.98 ± 6.18 cm (p = 0.273); for girls, the AHtSDS increased by 1.28 ± 1.48 (p = 0.000), and the disparity between AHt and THt was 3.64 ± 4.86 cm (n = 45, p = 0.000). (4) Subgroup analysis showed that, for boys with idiopathic short stature (ISS) and non-ISS, AHt SDS increased by 2.00 ± 1.16 (p = 0.000) and 0.71 ± 1.06 (p = 0.003) respectively, compared with the pre-treatment HtSDS; The disparities between AHt and THt were −0.70 ± 6.54 cm and 2.73 ± 5.37 cm respectively. For girls with ISS and non-ISS, AHtSDS increased by 2.73 ± 1.21 (p = 0.000) and 0.748 ± 1.19 (p = 0.001), respectively; AHt increased by 2.63 ± 6.12 cm (p = 0.165) and 4.02 ± 4.37 cm (p = 0.000) compared with THt, respectively. (5) Multiple linear regression analysis showed that the baseline bone age (BA) (β = −0.200, p = 0.003), basal IGF-1(β = −0.002, p = 0.008) and HtSDS (β = −0.679, p = 0.000) had negative effects on increment of AHtSDS.
Conclusion
For adolescents with normal pubertal onset and short stature, with or without ISS, GnRHa/rhGH therapy can effectively improve AHtSDS. After treatment, ISS adolescents can reach the THts, and Non-ISS adolescents can exceed their THts.




Similar content being viewed by others
References
M.J. Biehl, L.T. Raetzman, Developmental origins of hypothalamic cells controlling reproduction. Semin. Reprod. Med. 35, 121 (2017)
J. Karlberg, Secular trends in pubertal development. Horm. Res. 57(Suppl 2), 19 (2002)
G. Hajzadeh, N. Ghaemi, M.A. Hadjzadeh, S. Noroozi, N. Morovatdar, The effects of gonadotropin-releasing hormone analog and a combination of gonadotropin-releasing hormone analog and recombinant human growth hormone on adult height in girls with early puberty. Adv. Biomed. Res. 8, 57 (2019)
K. Yuen, S. Llahana, B.S. Miller, Adult growth hormone deficiency: clinical advances and approaches to improve adherence. Exp. Rev. Endocrinol. Metab. 1, 419–436 (2019)
G. Saggese, A.M. Pasquino, S. Bertelloni, G.I. Baroncelli, R. Battini, I. Pucarelli, M. Segni, G. Franchi, Effect of combined treatment with gonadotropin releasing hormone analogue and growth hormone in patients with central precocious puberty who had subnormal growth velocity and impaired height prognosis. Acta Paediatr. 84, 299 (1995)
M.S. Kim, H.J. Koh, G.Y. Lee, D.H. Kang, S.Y. Kim, Comparing adult height gain and menarcheal age between girls with central precocious puberty treated with gonadotropin-releasing hormone agonist alone and those treated with combined growth hormone therapy. Ann. Pediatr. Endocrinol. Metab. 24, 116 (2019)
M.K. Jung, K.C. Song, A.R. Kwon, H.W. Chae, D.H. Kim, H.S. Kim, Adult height in girls with central precocious puberty treated with gonadotropin-releasing hormone agonist with or without growth hormone. Ann. Pediatr. Endocrinol. Metab. 19, 214 (2014)
S. Liu, Q. Liu, X. Cheng, Y. Luo, Y. Wen, Effects and safety of combination therapy with gonadotropin-releasing hormone analogue and growth hormone in girls with idiopathic central precocious puberty: a meta-analysis. J. Endocrinol. Investig. 39, 1167 (2016)
A. Grimberg, D.B. Allen, Growth hormone treatment for growth hormone deficiency and idiopathic short stature: new guidelines shaped by the presence and absence of evidence. Curr. Opin. Pediatr. 29, 466 (2017)
N. Khawaja, H. Owaineh, A. Batieha, O. Frahid, The effect of gonadotropin-releasing hormone analogue on final adult height in children with idiopathic short stature. Med. Princ. Pract. 28, 509–516 (2019)
L. Lazar, S. Levy, T. Oron, J. Meyerovitch, L. de Vries, S. Shalitin, A. Tenenbaum, M. Phillip, Y. Lebenthal, The beneficial effect of combined GH/GnRHa therapy in increasing adult height outcome in children with ISS. J. Clin. Endocrinol. Metab. 104, 3287 (2019)
R. Lanes, P. Gunczler, Final height after combined growth hormone and gonadotrophin-releasing hormone analogue therapy in short healthy children entering into normally timed puberty. Clin. Endocrinol. 49, 197 (1998)
P. Cohen, A. Rogol, C. Deal, P. Saenger, E. Reiter, J. Ross, S. Chernausek, M. Savage, J. Wit, I.C.W. Participants, Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J. Clin. Endocrinol. Metab. 93, 4210 (2008)
J.L. Ross, P.A. Lee, R. Gut, J. Germak, Increased height standard deviation scores in response to growth hormone therapy to near-adult height in older children with delayed skeletal maturation: results from the ANSWER Program. Int.J. Pediatr. Endocrinol. 2015, 1–9 (2015)
W.W. Greulich, S.I. Pyle, Radiographic atlas of skeletal development of the hand and wrist. Am. J. Med. Sci. 130, 393–395 (1959)
N. Bayley, S.R. Pinneau, Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J. Pediatr. 40, 423 (1952)
D. Wu, G. Feng, C. Gong, Analysis of effect of growth hormone treatment on idiopathic short status children to near-adult height. J. Cap. Med. Univ. 39(1), 92–97 (2018)
A. Colmenares, L. Gonzalez, P. Gunczler, R. Lanes, Is the growth outcome of children with idiopathic short stature and isolated growth hormone deficiency following treatment with growth hormone and a luteinizing hormone-releasing hormone agonist superior to that obtained by GH alone? J. Pediatr. Endocrinol. Metab. 25, 651 (2012)
M.J. Kang, Novel genetic cause of idiopathic short stature. Ann. Pediatr. Endocrinol. Metab. 22, 153 (2017)
P. Cohen, G.M. Bright, A.D. Rogol, A.M. Kappelgaard, R.G. Rosenfeld, Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J. Clin. Endocrinol. Metab. 87, 90 (2002)
J.L. Ross, P.A. Lee, R. Gut, J. Germak, Attaining genetic height potential: analysis of height outcomes from the ANSWER Program in children treated with growth hormone over 5years. Growth Hormone IGF Res. 25, 286 (2015)
L. Sävendahl, O. Blankenstein, I. Oliver, H. Thybo Christesen, P. Lee, B. Tønnes Pedersen, V. Rakov, J. Ross, Gender influences short-term growth hormone treatment response in children. Horm. Res. Paediatr. 77, 188 (2012)
R. Balducci, V. Toscano, A. Mangiantini, G. Municchi, F. Vaccaro, S. Picone, A. Di Rito, B. Boscherini, Adult height in short normal adolescent girls treated with gonadotropin-releasing hormone analog and growth hormone. J. Clin. Endocrinol. Metab. 80, 3596 (1995)
G. Saggese, G. Cesaretti, S. Barsanti, A. Rossi, Combination treatment with growth hormone and gonadotropin-releasing hormone analogs in short normal girls. J. Pediatr. 126, 468 (1995)
L.E. Cohen, Idiopathic short stature: a clinical review. JAMA 311, 1787 (2014)
A. Deodati, S. Cianfarani, Impact of growth hormone therapy on adult height of children with idiopathic short stature: systematic review. BMJ 342, c7157 (2011)
L. Schena, C. Meazza, S. Pagani, V. Paganelli, E. Bozzola, C. Tinelli, F. Buzi, M. Bozzola, Efficacy of long-term growth hormone therapy in short non-growth hormone-deficient children. J. Pediatr. Endocrinol. Metab. 30, 197 (2017)
J.M. Wit, P.E. Clayton, A.D. Rogol, M.O. Savage, P.H. Saenger, P. Cohen, Idiopathic short stature: definition, epidemiology, and diagnostic evaluation. Growth Horm. IGF Res. 18, 89 (2008)
Y.Q. Ying, L. Hou, Y. Liang, W. Wu, X.P. Luo, Efficacy and safety of recombinant human growth hormone in treating Chinese children with idiopathic short stature. Growth Horm IGF Res 42–43, 80 (2018)
J.S. Felício, L.C. Janaú, M.A. Moraes, N.A. Zahalan, F. de Souza Resende, M.N. de Lemos, N.J.K. de Souza Neto, I.I. Farias De Franco, L.T.C. Leitão, L.D.S.D. Silva, M.C.N.I. de Oliveira, A.L. de Alcântara, A.C. Contente Braga De Souza, W.M. Da Silva, M.C. Dos Santos, N.N.M. de Queiroz, L.V. de Moraes, A.B. de Figueiredo, A.L.P. Farinassi, L.M.D.C. Farias, D.D. Da Silva, K.M. Felício, J.F. Abrahão Neto, Diagnosis of idiopathic GHD in children based on response to rhGH treatment: the importance of GH provocative tests and IGF-1. Front. Endocrinol. 10, 638 (2019)
P.V. Borras, J.P. Lopez-Siguero, G. Martinez, R. Corripio, J.M. Fernandez, J.I. Labarta, M. Ferrer, N. Cabrinety, P. Prieto, M. Ramon-Krauel, J. Bosch, R. Espino, G.M. Palla, F.J. Rebollo, A follow-up study to monitor adult height among Spanish children with growth hormone deficiency who received biosimilar human recombinant growth hormone (Omnitrope(R)) during a phase III clinical trial. Adv Ther. 32, 148 (2015)
R. Horikawa, T. Ogata, Y. Matsubara, S. Yokoya, Y. Ogawa, K. Nishijima, T. Endo, K. Ozono, Long-term efficacy and safety of two doses of Norditropin((R)) (somatropin) in Noonan syndrome: a 4-year randomized, double-blind, multicenter trial in Japanese patients. Endocr. J. (2020). https://doi.org/10.1507/endocrj.EJ19-0371. Online ahead of print
A.M. Pasquino, I. Pucarelli, M. Roggini, M. Segni, Adult height in short normal girls treated with gonadotropin-releasing hormone analogs and growth hormone. J. Clin. Endocrinol. Metab. 85, 619 (2000)
J.M. Wit, H.V. Balen, G.A. Kamp, W. Oostdijk, Benefit of postponing normal puberty for improving final height. Eur. J. Endocrinol. 151(Suppl 1), S41 (2004)
R. Lindsay, M. Feldkamp, D. Harris, J. Robertson, M. Rallison, Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J. Pediatr. 125, 29 (1994)
A. Grimberg, G.P. Kanter, U.S. Growth, Hormone use in the idiopathic short stature era: trends in insurer payments and patient financial burden. J. Endocr. Soc. 3, 2023 (2019)
M. Chandar, P.B. Kaplowitz, P. Vaidyanathan, Challenges of securing growth hormone coverage for idiopathic short stature: review of the 7-year experience at one institution. Endocr. Pract. 25, 156 (2019)
J.A. Yanovski, S.R. Rose, G. Municchi, O.H. Pescovitz, S.C. Hill, F.G. Cassorla, G.J. Cutler, Treatment with a luteinizing hormone-releasing hormone agonist in adolescents with short stature. N. Engl. J. Med. 348, 908 (2003)
D. Al-Abdulrazzaq, A. Al-Taiar, K. Hassan, I. Al-Basari, Recombinant growth hormone therapy in children with short stature in Kuwait: a cross-sectional study of use and treatment outcomes. BMC Endocr. Disord. 15, 76 (2015)
S. Pedicelli, E. Peschiaroli, E. Violi, S. Cianfarani, Controversies in the definition and treatment of idiopathic short stature (ISS). J. Clin. Res. Pediatr. Endocrinol. 1, 105 (2009)
A.L. Rosenbloom, Idiopathic short stature: conundrums of definition and treatment. Int. J. Pediatr. Endocrinol. 2009, 470378 (2009)
W. Hogler, J. Briody, B. Moore, P.W. Lu, C.T. Cowell, Effect of growth hormone therapy and puberty on bone and body composition in children with idiopathic short stature and growth hormone deficiency. Bone 37, 642 (2005)
G.A. Kamp, J.J. Waelkens, K.S. de Muinck, D.W.H. Delemarre-Van, L. Verhoeven-Wind, A.H. Zwinderman, J.M. Wit, High dose growth hormone treatment induces acceleration of skeletal maturation and an earlier onset of puberty in children with idiopathic short stature. Arch. Dis. Child. 87, 215 (2002)
V. Mericq, H. Gajardo, M. Eggers, A. Avila, F. Cassorla, Effects of treatment with GH alone or in combination with LHRH analog on bone mineral density in pubertal GH-deficient patients. J. Clin. Endocrinol. Metab. 87, 84 (2002)
J.H. Quitmann, M. Bullinger, R. Sommer, A.C. Rohenkohl, Bernardino Da, N.M. Silva, Associations between psychological problems and quality of life in pediatric short stature from patients’ and parents’ perspectives. PLoS ONE. 11, e153953 (2016)
X.C.R.E.G. Ge, The relationship between pubertal transition and psychological distress among adolescent boys. J. Res. Adolesc. 11, 49 (2001)
B.H. Visser-van, R. Geenen, M. Moerbeek, R. Stroop, G.A. Kamp, J. Huisman, J.M. Wit, G. Sinnema, Psychosocial functioning of adolescents with idiopathic short stature or persistent short stature born small for gestational age during three years of combined growth hormone and gonadotropin-releasing hormone agonist treatment. Horm. Res. 64, 77 (2005)
B.H. Visser-van, R. Geenen, G.A. Kamp, J. Huisman, J.M. Wit, G. Sinnema, Long-term psychosocial consequences of hormone treatment for short stature. Acta Paediatr. 96, 715 (2007)
Funding
This study was funded by the National Key Research and Development Program of China (2016YFC0905102, 2016YFA0101003), CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-002, 2017-I2M-3-007), and the Project of National Natural Science Foundation of China (81771576).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Wang, X., Zhao, Y. et al. Combined therapy with GnRH analogue and growth hormone increases adult height in children with short stature and normal pubertal onset. Endocrine 69, 615–624 (2020). https://doi.org/10.1007/s12020-020-02375-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02375-5