Abstract
Purpose
To describe the experience with radioiodine-resistant differentiated thyroid cancer (RR-DTC) patients treated with lenvatinib in two university hospitals from Argentina.
Methods
Adult patients with a diagnosis of RR-DTC treated with lenvatinib from April 2017 to February 2020 were registered into a retrospective database. Primary objectives were assessment of progression-free survival (PFS) and tumor response evaluated according to RECIST v 1.1. Adverse events (AEs) were evaluated by using Common Terminology Criteria for Adverse Events v5.0.
Results
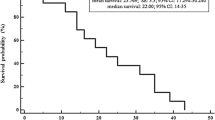
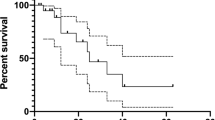
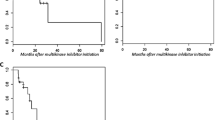
Twenty-two patients were treated with lenvatinib, 13 of whom had previously received one or more multikinase inhibitors. Median duration of treatment was 7.1 months (2.2–24). Best overall response was complete response in one patient (4.5%), partial response in seven (31.8%), stable disease in seven (31.8%), and progressive disease in six (27.3%). Median PFS was 13.7 months (95% CI 3.2–24.2). All patients experienced at least one AE. Grade ≥3 AEs were observed in eight (36.4%) patients. Hypertension was the most frequent AE (63.6%) and the most common grade ≥3 AE (22.7%). Definitive withdrawal was necessary in two patients due to recurrent proteinuria (9%).
Conclusions
Tumor responses and PFS in our study were in line with other real-life clinical data and they seem to be inferior to the reported in the SELECT trial, probably related to the higher number of patients with prior MKI therapy, comorbidities, and poor performance status. Although virtually all patients experienced AEs, most of them were manageable and rarely a definitive withdrawal was necessary.



Similar content being viewed by others
References
J. Matsui, Y. Yamamoto, Y. Funahashi, A. Tsuruoka, T. Watanabe, T. Wakabayashi, T. Uenaka, M. Asada, E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer 122, 664–671 (2008)
M. Schlumberger, M. Tahara, L.J. Wirth, B. Robinson, M.S. Brose, R. Elisei, M.A. Habra, K. Newbold, M.H. Shah, A.O. Hoff, A.G. Gianoukakis, N. Kiyota, M.H. Taylor, S.B. Kim, M.K. Krzyzanowska, C.E. Dutcus, B. de las Heras, J. Zhu, S.I. Sherman, Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 (2015)
M. Schlumberger, M. Brose, R. Elisei, S. Leboulleux, M. Luster, F. Pitoia, F. Pacini, Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2, 356–358 (2014)
B.R. Haugen, E.K. Alexander, K.C. Bible, G. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G. Randolph, A. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016)
E.A. Eisenhauer, P. Therasse, J. Bogaerts, L.H. Schwartz, D. Sargent, R. Ford, J. Dancey, S. Arbuck, S. Gwyther, M. Mooney, L. Rubinstein, L. Shankar, L. Dodd, R. Kaplan, D. Lacombe, J. Verweij, New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009)
National Cancer Institute, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 15 Jan 2020
A. Berdelou, I. Borget, Y. Godbert, T. Nguyen, M.E. Garcia, C.N. Chougnet, A. Ferru, C. Buffet, O. Chabre, O. Huillard, S. Leboulleux, M. Schlumberger, Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid 28, 72–78 (2018)
S. Jasim, N.M. Iniguez-Ariza, C.R. Hilger, A.V. Chintakuntlawar, M.M. Ryder, J.C. Morris 3rd, K.C. Bible, Optimizing lenvatinib therapy in patients with metastatic radioactive iodine-resistant differentiated thyroid cancers. Endocr. Pract. 23, 1254–1261 (2017)
K. Sugino, M. Nagahama, W. Kitagawa, K. Ohkuwa, T. Uruno, K. Matsuzu, A. Suzuki, C. Masaki, J. Akaishi, K.Y. Hames, C. Tomoda, Y. Ogimi, K. Ito, Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr. J. 65, 299–306 (2018)
L.D. Locati, A. Piovesan, C. Durante, M. Bregni, M.G. Castagna, S. Zovato, M. Giusti, T. Ibrahim, E. Puxeddu, G. Fedele, G. Pellegriti, G. Rinaldi, D. Giuffrida, F. Verderame, F. Bertolini, C. Bergamini, A. Nervo, G. Grani, S. Rizzati, S. Morelli, I. Puliafito, R. Elisei, Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur. J. Cancer 118, 35–40 (2019)
E.K. Lee, S.M. Kim, B.H. Kim, M.J. Kim, D.J. Lim, M.H. Kim, D.Y. Shin, H.C. Kang, B.C. Ahn, S.W. Kim, H.Y. Ahn, Y.J. Park, Lesion-based evaluation predicts treatment response to lenvatinib for radioactive iodine-refractory differentiated thyroid cancer: a Korean Multicenter Retrospective Study. Thyroid 29, 1811–1819 (2019)
C. Masaki, K. Sugino, N. Saito, J. Akaishi, K. Hames, C. Tomoda, A. Suzuki, K. Matsuzu, T. Uruno, K. Ohkuwa, W. Kitagawa, M. Nagahama, K. Ito, Efficacy and limitations of lenvatinib therapy for radioiodine-refractory differentiated thyroid cancer: real-world experiences. Thyroid. 30, 214–221 (2020)
M. D. Aydemirli, E. Kapiteijn, K. R. M. Ferrier, N. Ottevanger, T. P. Links, A. N. A. van der Horst-Schrivers, K. E. Broekman, R. H. H. Groenwold, J. Zwaveling, Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data. Eur. J. Endocrinol. 182, 131–138 (2020)
H. Yamazaki, H. Iwasaki, H. Takasaki, N. Suganuma, R. Sakai, K. Masudo, H. Nakayama, Y. Rino, M. Masuda, Efficacy and tolerability of initial low-dose lenvatinib to treat differentiated thyroid cancer. Medicine 98, e14774 (2019)
M. Tahara, M.S. Brose, L.J. Wirth, T. Suzuki, H. Miyagishi, K. Fujino, C.E. Dutcus, A. Gianoukakis, Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur. J. Cancer 106, 61–68 (2019)
C. Suzuki, N. Kiyota, Y. Imamura, H. Goto, H. Suto, N. Chayahara, M. Toyoda, Y. Ito, A. Miya, A. Miyauchi, N. Otsuki, K.I. Nibu, H. Minami, Exploratory analysis of prognostic factors for lenvatinib in radioiodine-refractory differentiated thyroid cancer. Head. Neck. 41, 3023–3032 (2019)
M.S. Brose, F.P. Worden, K.L. Newbold, M. Guo, A. Hurria, Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J. Clin. Oncol. 35, 2692–2699 (2017)
M.E. Cabanillas, S. Takahashi, Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin. Oncol. 46, 57–64 (2019)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F.P. is medical advisor, speaker and Steering Committee Bayer. Consultancy for Biotoscana and Raffo Laboratories. I.C. is speaker for Bayer and Raffo. Consultancy for Biotoscana. J.M.C. received consultancy fees for Biotoscana. The rest of the authors have no conflicts of interests to declare.
Ethics approval
The study was approved by institutional review board.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jerkovich, F., Califano, I., Bueno, F. et al. Real-life use of lenvatinib in patients with differentiated thyroid cancer: experience from Argentina. Endocrine 69, 142–148 (2020). https://doi.org/10.1007/s12020-020-02290-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02290-9