Abstract
Purpose
An increasing body of evidence suggests that dipeptidyl-peptidase 4 (DPP-4) inhibitors could play a role in the development of bullous pemphigoid. The knowledge regarding this association is based on case reports, pharmacovigilance database analyses, and observational studies. Data from randomized clinical trials are a relevant source of information on adverse events. Since no single trial has a sufficient power to assess the risk of very rare adverse events, such as pemphigoid, metanalyses of RCTs could be a useful tool for exploring this issue.
Methods
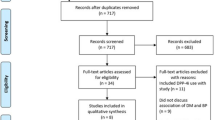
An extensive Medline, Embase and Cochrane Database search for sitagliptin or vildagliptin, omarigliptin or saxagliptin or alogliptin or trelagliptin or anagliptin or linagliptin or gemigliptin or evogliptin or teneligliptin was performed up to September 30th, 2019. All trials performed on type 2 diabetes, with duration ≥24 weeks, and comparing DPP4i with placebo or active drugs were collected. The study has been registered on PROSPERO (#153344). Mantel-Haenszel odds ratio (MH-OR) with 95% Confidence Interval (95% CI) was calculated for pemphigoid.
Results
A total of 138 eligible trials were identified (61,514 patients in DPP-4 inhibitors and 59,661 patients in the control group). Only six trials reported at least one case of pemphigoid (17 and 1 cases in DPP4i and control groups, respectively). DPP-4 inhibitors were associated with an increased risk of pemphigoid (MH-OR 4.44 [1.31, 15.00], p = 0.020). A separate analysis for trials with linagliptin showed a significant increase of BP with the active drug (MH-OR 4.69 [1.09, 20.22]; p = 0.04).
Conclusions
In conclusion, available data from randomized controlled trials seem to confirm the association between DPP-4 inhibitors and bullous pemphigoid. This association could be limited to one molecule of the class (i.e., linagliptin), although data on other DPP4-i (e.g., vildagliptin) are insufficient to rule out similar detrimental effects.

Similar content being viewed by others
References
K. Kridin, D. Kneiber, E.H. Kowalski, M. Valdebran, K.T. Amber, Epidermolysis bullosa acquisita: a comprehensive review. Autoimmun. Rev. 18(8), 786–795 (2019)
S. Bastuji-Garin, P. Joly, C. Picard-Dahan, P. Bernard, L. Vaillant, C. Pauwels et al. Drugs associated with bullous pemphigoid. A case-control study. Arch. Dermatol. 132(3), 272–276 (1996)
A. Lloyd-Lavery, C.C. Chi, F. Wojnarowska, K. Taghipour, The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 149(1), 58–62 (2013)
C.W. Tan, Y. Pang, B. Sim, T. Thirumoorthy, S.M. Pang, H.Y. Lee, The association between drugs and bullous pemphigoid. Br. J. Dermatol. 176(2), 549–551 (2017)
J. Bene, G. Moulis, I. Bennani, M. Auffret, P. Coupe, S. Babai et al. Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case-noncase study in the French Pharmacovigilance database. Br. J. Dermatol. 175(2), 296–301 (2016)
C. Carnovale, F. Mazhar, E. Arzenton, U. Moretti, M. Pozzi, G. Mosini, et al. Bullous pemphigoid induced by dipeptidyl peptidase-4 (DPP-4) inhibitors: a pharmacovigilance-pharmacodynamic/pharmacokinetic assessment through an analysis of the vigibase(R). Expert Opin. Drug Saf. 18(11), 1099–1108 (2019)
A. Reolid, E. Munoz-Aceituno, P. Rodriguez-Jimenez, E. Gonzalez-Rojano, M. Llamas-Velasco, J. Fraga, et al. Bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors. A case series and analysis of cases reported in the Spanish pharmacovigilance database. Int. J. Dermatol. 59(2), 197–206 (2019)
M. Benzaquen, L. Borradori, P. Berbis, S. Cazzaniga, R. Valero, M.A. Richard et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J. Am. Acad. Dermatol. 78(6), 1090–1096 (2018)
A. Douros, J. Rouette, H. Yin, O.H.Y. Yu, K.B. Filion, L. Azoulay, Dipeptidyl Peptidase 4 Inhibitors and the Risk of Bullous Pemphigoid Among Patients With Type 2 Diabetes. Diabet. Care 42(8), 1496–1503 (2019)
S.H. Lee, I. Gantz, E. Round, M. Latham, E.A. O’Neill, P. Ceesay et al. A randomized, placebo-controlled clinical trial evaluating the safety and efficacy of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus inadequately controlled by glimepiride and metformin. BMC Endocr. Disord. 17(1), 70 (2017)
O. Varpuluoma, A.K. Forsti, J. Jokelainen, M. Turpeinen, M. Timonen, L. Huilaja et al. Vildagliptin significantly increases the risk of bullous pemphigoid: a Finnish nationwide registry study. J. Investig. Dermatol. 138(7), 1659–1661 (2018)
K. Kridin, A. D. Cohen, Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid: a systematic review and meta-analysis. J. Am. Acad. Dermatol. (2018) https://doi.org/10.1016/j.jaad.2018.09.048 (Epub-ahead of print)
T.Y. Chuang, W. Korkij, K. Soltani, J. Clayman, J. Cook, Increased frequency of diabetes mellitus in patients with bullous pemphigoid: a case-control study. J. Am. Acad. Dermatol. 11(6), 1099–1102 (1984)
D. Moher, A. Liberati, J. Tetzlaff, D.G.J.Aoim. Altman, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.151(4), 264–269 (2009)
Funding
This research was performed as a part of the institutional activity of the unit, with no specific funding. All expenses, including salaries of the investigators, were covered by public research funds assigned to the unit. The paper was drafted and revised by the authors in accordance with ICJME standards for authorship. The corresponding author had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
M.M and E.M were involved in each of the following points: 1. Design 2. Data Collection 3. Analysis 4. Writing paper. I.D, G.A.S, B.N, and C.M were involved in each of the following points: 1. Data Collection 2. Paper revision.
Corresponding author
Ethics declarations
Conflict of interest
G.A.S and I.D have received speaking fees from Astra Zeneca, Novonordisk; C.M has no conflict of interest; B.N is presently employee of Novo Nordisk; M.M has received speaking fees from Astra Zeneca, Bristol Myers Squibb, Boehringer-Ingelheim, Eli-Lilly, Merck, Novo Nordisk, Sanofi, and Novartis and research grants from Bristol Myers Squibb; E.M has received consultancy fees from Merck and Novartis speaking fees from Astra Zeneca, Bristol Myers Squibb, Boehringer-Ingelheim, Eli-Lilly, Merck, Novo Nordisk, Sanofi, and Novartis and research grants from Merck, Novartis, and Takeda. All the authors approved the final version of this paper. M.M is the person who takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Silverii, G.A., Dicembrini, I., Nreu, B. et al. Bullous pemphigoid and dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized controlled trials. Endocrine 69, 504–507 (2020). https://doi.org/10.1007/s12020-020-02272-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02272-x