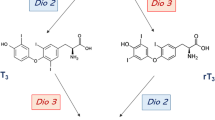
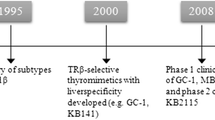
Abstract
Several metabolic products that derive from l-thyroxine (T4) and 3,3′5-l-triiodothyronine (T3), the main thyroid hormones secreted by the thyroid gland, possess biologic activities. Among these metabolites or derivatives showing physiological actions some have received greater attention: diiodothyronines, iodothyronamines, acetic acid analogues. It is known that increased thyroid hormone (T3 and T4) levels can improve serum lipid profiles and reduce body fat. These positive effects are, however, counterbalanced by adverse effects on the heart, muscle and bone, limiting their use. In addition to the naturally occurring metabolites, thyroid hormone analogues have been developed that either have selective effects on specific tissues or bind selectively to thyroid hormone receptor (TR) isoform. Among these GC-1, KB141, KB2115, and DITPA were deeply investigated and displayed promising therapeutic results in the potential treatment of conditions such as dyslipidemias and obesity. In this review, we summarize the current knowledge of metabolites and analogues of T4 and T3 with reference to their possible clinical application in the treatment of human diseases.
Similar content being viewed by others
References
M.A. Kowalik, A. Columbano, A. Perra, Thyroid hormones, thyromimetics and their metabolites in the treatment of liver disease. Front Endocrinol. (Lausanne) 9, 382 (2018). https://doi.org/10.3389/fendo.2018.00382
G. Chiellini, J.W. Apriletti, H.A. Yoshihara, J.D. Baxter, R.C. Ribeiro, T.S. Scanlan, A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem. Biol. 5(6), 299–306 (1998)
S.U. Trost, E. Swanson, B. Gloss, D.B. Wang-Iverson, H. Zhang, T. Volodarsky, G.J. Grover, J.D. Baxter, G. Chiellini, T.S. Scanlan, W.H. Dillmann, The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 141(9), 3057–3064 (2000). https://doi.org/10.1210/endo.141.9.7681
L. Johansson, M. Rudling, T.S. Scanlan, T. Lundasen, P. Webb, J. Baxter, B. Angelin, P. Parini, Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc. Natl Acad. Sci. USA 102(29), 10297–10302 (2005). https://doi.org/10.1073/pnas.0504379102
J.Z. Lin, A.J. Martagon, W.A. Hsueh, J.D. Baxter, J.A. Gustafsson, P. Webb, K.J. Phillips, Thyroid hormone receptor agonists reduce serum cholesterol independent of the LDL receptor. Endocrinology 153(12), 6136–6144 (2012). https://doi.org/10.1210/en.2011-2081
A. Goncalves, C.C. Tolentino, F.R. Souza, J.C. Huss, L. Zinato Kde, L.T. Lopes, R. Furlanetto Junior, A. Neves Fde, The thyroid hormone receptor beta-selective agonist GC-1 does not affect tolerance to exercise in hypothyroid rats. Arch. Endocrinol. Metab. 59(2), 141–147 (2015). https://doi.org/10.1590/2359-3997000000027
L. Ye, Y.L. Li, K. Mellstrom, C. Mellin, L.G. Bladh, K. Koehler, N. Garg, A.M. Garcia Collazo, C. Litten, B. Husman, K. Persson, J. Ljunggren, G. Grover, P.G. Sleph, R. George, J. Malm, Thyroid receptor ligands. 1. Agonist ligands selective for the thyroid receptor beta1. J. Med Chem. 46(9), 1580–1588 (2003). https://doi.org/10.1021/jm021080f
G.J. Grover, K. Mellstrom, L. Ye, J. Malm, Y.L. Li, L.G. Bladh, P.G. Sleph, M.A. Smith, R. George, B. Vennstrom, K. Mookhtiar, R. Horvath, J. Speelman, D. Egan, J.D. Baxter, Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc. Natl Acad. Sci. USA 100(17), 10067–10072 (2003). https://doi.org/10.1073/pnas.1633737100
A. Berkenstam, J. Kristensen, K. Mellstrom, B. Carlsson, J. Malm, S. Rehnmark, N. Garg, C.M. Andersson, M. Rudling, F. Sjoberg, B. Angelin, J.D. Baxter, The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc. Natl Acad. Sci. USA 105(2), 663–667 (2008). https://doi.org/10.1073/pnas.0705286104
D.F. Vatner, D. Weismann, S.A. Beddow, N. Kumashiro, D.M. Erion, X.H. Liao, G.J. Grover, P. Webb, K.J. Phillips, R.E. Weiss, J.S. Bogan, J. Baxter, G.I. Shulman, V.T. Samuel, Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am. J. Physiol. Endocrinol. Metab. 305(1), E89–E100 (2013). https://doi.org/10.1152/ajpendo.00573.2012
P.W. Ladenson, J.D. Kristensen, E.C. Ridgway, A.G. Olsson, B. Carlsson, I. Klein, J.D. Baxter, B. Angelin, Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N. Engl. J. Med 362(10), 906–916 (2010). https://doi.org/10.1056/NEJMoa0905633
Bio, K.: Karo Bio terminates the eprotirome program. (2010). http://www.karobio.com/investormedia/pressreleaser/pressrelease?pid=639535
B. Sjouke, G. Langslet, R. Ceska, S.J. Nicholls, S.E. Nissen, M. Ohlander, P.W. Ladenson, A.G. Olsson, G.K. Hovingh, J.J. Kastelein, Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): a randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol. 2(6), 455–463 (2014). https://doi.org/10.1016/S2213-8587(14)70006-3
R. Taub, E. Chiang, M. Chabot-Blanchet, M.J. Kelly, R.A. Reeves, M.C. Guertin, J.C. Tardif, Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-beta agonist. Atherosclerosis 230(2), 373–380 (2013). https://doi.org/10.1016/j.atherosclerosis.2013.07.056
G.D. Pennock, T.E. Raya, J.J. Bahl, S. Goldman, E. Morkin, Cardiac effects of 3,5-diiodothyropropionic acid, a thyroid hormone analog with inotropic selectivity. J. Pharm. Exp. Ther. 263(1), 163–169 (1992)
S. Goldman, M. McCarren, E. Morkin, P.W. Ladenson, R. Edson, S. Warren, J. Ohm, H. Thai, L. Churby, J. Barnhill, T. O’Brien, I. Anand, A. Warner, B. Hattler, M. Dunlap, J. Erikson, M.C. Shih, P. Lavori, DITPA (3,5-Diiodothyropropionic Acid), a thyroid hormone analog to treat heart failure: phase II trial veterans affairs cooperative study. Circulation 119(24), 3093–3100 (2009). https://doi.org/10.1161/CIRCULATIONAHA.108.834424
P.W. Ladenson, M. McCarren, E. Morkin, R.G. Edson, M.C. Shih, S.R. Warren, J.G. Barnhill, L. Churby, H. Thai, T. O’Brien, I. Anand, A. Warner, B. Hattler, M. Dunlap, J. Erikson, S. Goldman, Effects of the thyromimetic agent diiodothyropropionic acid on body weight, body mass index, and serum lipoproteins: a pilot prospective, randomized, controlled study. J. Clin. Endocrinol. Metab. 95(3), 1349–1354 (2010). https://doi.org/10.1210/jc.2009-1209
C. Di Cosmo, X.H. Liao, A.M. Dumitrescu, R.E. Weiss, S. Refetoff, A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology 150(9), 4450–4458 (2009). https://doi.org/10.1210/en.2009-0209
A.M. Ferrara, X.H. Liao, H. Ye, R.E. Weiss, A.M. Dumitrescu, S. Refetoff, The thyroid hormone analog DITPA ameliorates metabolic parameters of male mice with Mct8 deficiency. Endocrinology 156(11), 3889–3894 (2015). https://doi.org/10.1210/en.2015-1234
C.F. Verge, D. Konrad, M. Cohen, C. Di Cosmo, A.M. Dumitrescu, T. Marcinkowski, S. Hameed, J. Hamilton, R.E. Weiss, S. Refetoff, Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J. Clin. Endocrinol. Metab. 97(12), 4515–4523 (2012). https://doi.org/10.1210/jc.2012-2556
M.B. Dratman, On the mechanism of action of thyroxin, an amino acid analog of tyrosine. J. Theor. Biol. 46(1), 255–270 (1974)
C.S. Hoefig, T. Wuensch, E. Rijntjes, I. Lehmphul, H. Daniel, U. Schweizer, J. Mittag, J. Kohrle, Biosynthesis of 3-Iodothyronamine from T4 in murine intestinal tissue. Endocrinology 156(11), 4356–4364 (2015). https://doi.org/10.1210/en.2014-1499
C.S. Hoefig, J. Kohrle, G. Brabant, K. Dixit, B. Yap, C.J. Strasburger, Z. Wu, Evidence for extrathyroidal formation of 3-iodothyronamine in humans as provided by a novel monoclonal antibody-based chemiluminescent serum immunoassay. J. Clin. Endocrinol. Metab. 96(6), 1864–1872 (2011). https://doi.org/10.1210/jc.2010-2680
T.S. Scanlan, K.L. Suchland, M.E. Hart, G. Chiellini, Y. Huang, P.J. Kruzich, S. Frascarelli, D.A. Crossley, J.R. Bunzow, S. Ronca-Testoni, E.T. Lin, D. Hatton, R. Zucchi, D.K. Grandy, 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med 10(6), 638–642 (2004). https://doi.org/10.1038/nm1051
A. Saba, G. Chiellini, S. Frascarelli, M. Marchini, S. Ghelardoni, A. Raffaelli, M. Tonacchera, P. Vitti, T.S. Scanlan, R. Zucchi, Tissue distribution and cardiac metabolism of 3-iodothyronamine. Endocrinology 151(10), 5063–5073 (2010). https://doi.org/10.1210/en.2010-0491
V. Mariotti, E. Melissari, C. Iofrida, M. Righi, M. Di Russo, R. Donzelli, A. Saba, S. Frascarelli, G. Chiellini, R. Zucchi, S. Pellegrini, Modulation of gene expression by 3-iodothyronamine: genetic evidence for a lipolytic pattern. PLoS ONE 9(11), e106923 (2014). https://doi.org/10.1371/journal.pone.0106923
M.E. Manni, G. De Siena, A. Saba, M. Marchini, E. Landucci, E. Gerace, M. Zazzeri, C. Musilli, D. Pellegrini-Giampietro, R. Matucci, R. Zucchi, L. Raimondi, Pharmacological effects of 3-iodothyronamine (T1AM) in mice include facilitation of memory acquisition and retention and reduction of pain threshold. Br. J. Pharm. 168(2), 354–362 (2013). https://doi.org/10.1111/j.1476-5381.2012.02137.x
M. Rogowski, L. Gollahon, G. Chellini, F.M. Assadi-Porter, Uptake of 3-iodothyronamine hormone analogs inhibits the growth and viability of cancer cells. FEBS Open Bio 7(4), 587–601 (2017). https://doi.org/10.1002/2211-5463.12205
F. Goglia, J. Torresani, P. Bugli, A. Barletta, G. Liverini, In vitro binding of triiodothyronine to rat liver mitochondria. Pflug. Arch. 390(2), 120–124 (1981)
C. Horst, H. Rokos, H.J. Seitz, Rapid stimulation of hepatic oxygen consumption by 3,5-di-iodo-L-thyronine. Biochem J. 261(3), 945–950 (1989). https://doi.org/10.1042/bj2610945
R. Senese, P. de Lange, G. Petito, M. Moreno, F. Goglia, A. Lanni, 3,5-Diiodothyronine: a novel thyroid hormone metabolite and potent modulator of energy metabolism. Front Endocrinol. (Lausanne) 9, 427 (2018). https://doi.org/10.3389/fendo.2018.00427
R.A. Louzada, D.P. Carvalho, Similarities and differences in the peripheral actions of thyroid hormones and their metabolites. Front Endocrinol. (Lausanne) 9, 394 (2018). https://doi.org/10.3389/fendo.2018.00394
G. Sacripanti, N.M. Nguyen, L. Lorenzini, S. Frascarelli, A. Saba, R. Zucchi, S. Ghelardoni, 3,5-Diiodo-l-thyronine increases glucose consumption in cardiomyoblasts without affecting the contractile performance in rat heart. Front Endocrinol. (Lausanne) 9, 282 (2018). https://doi.org/10.3389/fendo.2018.00282
M. Moreno, A. Lombardi, L. Beneduce, E. Silvestri, G. Pinna, F. Goglia, A. Lanni, Are the effects of T3 on resting metabolic rate in euthyroid rats entirely caused by T3 itself? Endocrinology 143(2), 504–510 (2002). https://doi.org/10.1210/endo.143.2.8613
I. Lehmphul, G. Brabant, H. Wallaschofski, M. Ruchala, C.J. Strasburger, J. Kohrle, Z. Wu, Detection of 3,5-diiodothyronine in sera of patients with altered thyroid status using a new monoclonal antibody-based chemiluminescence immunoassay. Thyroid 24(9), 1350–1360 (2014). https://doi.org/10.1089/thy.2013.0688
P.J. Davis, F. Goglia, J.L. Leonard, Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 12(2), 111–121 (2016). https://doi.org/10.1038/nrendo.2015.205
M. Moreno, A. Giacco, C. Di Munno, F. Goglia, Direct and rapid effects of 3,5-diiodo-L-thyronine (T2). Mol. Cell Endocrinol. 458, 121–126 (2017). https://doi.org/10.1016/j.mce.2017.02.012
P. Navarrete-Ramirez, M. Luna, R.C. Valverde, A. Orozco, 3,5-di-iodothyronine stimulates tilapia growth through an alternate isoform of thyroid hormone receptor beta1. J. Mol. Endocrinol. 52(1), 1–9 (2014). https://doi.org/10.1530/JME-13-0145
A. Lanni, M. Moreno, A. Lombardi, F. Goglia, 3,5-Diiodo-L-thyronine and 3,5,3’-triiodo-L-thyronine both improve the cold tolerance of hypothyroid rats, but possibly via different mechanisms. Pflug. Arch. 436(3), 407–414 (1998). https://doi.org/10.1007/s004240050650
A. Lombardi, R. De Matteis, M. Moreno, L. Napolitano, R.A. Busiello, R. Senese, P. de Lange, A. Lanni, F. Goglia, Responses of skeletal muscle lipid metabolism in rat gastrocnemius to hypothyroidism and iodothyronine administration: a putative role for FAT/CD36. Am. J. Physiol. Endocrinol. Metab. 303(10), E1222–E1233 (2012). https://doi.org/10.1152/ajpendo.00037.2012
E. Silvestri, A. Lombardi, M. Coppola, A. Gentile, F. Cioffi, R. Senese, F. Goglia, A. Lanni, M. Moreno, P. de Lange, Differential effects of 3,5-diiodo-l-thyronine and 3,5,3′-triiodo-l-thyronine on mitochondrial respiratory pathways in liver from hypothyroid rats. Cell Physiol. Biochem 47(6), 2471–2483 (2018). https://doi.org/10.1159/000491620
A. Lanni, M. Moreno, A. Lombardi, P. de Lange, E. Silvestri, M. Ragni, P. Farina, G.C. Baccari, P. Fallahi, A. Antonelli, F. Goglia, 3,5-diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 19(11), 1552–1554 (2005). https://doi.org/10.1096/fj.05-3977fje
P. de Lange, F. Cioffi, R. Senese, M. Moreno, A. Lombardi, E. Silvestri, R. De Matteis, L. Lionetti, M.P. Mollica, F. Goglia, A. Lanni, Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes 60(11), 2730–2739 (2011). https://doi.org/10.2337/db11-0207
E. Silvestri, F. Cioffi, D. Glinni, M. Ceccarelli, A. Lombardi, P. de Lange, A. Chambery, V. Severino, A. Lanni, F. Goglia, M. Moreno, Pathways affected by 3,5-diiodo-l-thyronine in liver of high fat-fed rats: evidence from two-dimensional electrophoresis, blue-native PAGE, and mass spectrometry. Mol. Biosyst. 6(11), 2256–2271 (2010). https://doi.org/10.1039/c0mb00040j
M. Moreno, E. Silvestri, R. De Matteis, P. de Lange, A. Lombardi, D. Glinni, R. Senese, F. Cioffi, A.M. Salzano, A. Scaloni, A. Lanni, F. Goglia, 3,5-Diiodo-L-thyronine prevents high-fat-diet-induced insulin resistance in rat skeletal muscle through metabolic and structural adaptations. FASEB J. 25(10), 3312–3324 (2011). https://doi.org/10.1096/fj.11-181982
M.P. Mollica, L. Lionetti, M. Moreno, A. Lombardi, P. De Lange, A. Antonelli, A. Lanni, G. Cavaliere, A. Barletta, F. Goglia, 3,5-diiodo-l-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J. Hepatol. 51(2), 363–370 (2009). https://doi.org/10.1016/j.jhep.2009.03.023
E. Grasselli, A. Voci, I. Demori, G. Vecchione, A.D. Compalati, G. Gallo, F. Goglia, R. De Matteis, E. Silvestri, L. Vergani, Triglyceride Mobilization from lipid droplets sustains the anti-steatotic action of iodothyronines in cultured rat hepatocytes. Front Physiol. 6, 418 (2015). https://doi.org/10.3389/fphys.2015.00418
E. Grasselli, A. Voci, I. Demori, L. Canesi, R. De Matteis, F. Goglia, A. Lanni, G. Gallo, L. Vergani, 3,5-Diiodo-L-thyronine modulates the expression of genes of lipid metabolism in a rat model of fatty liver. J. Endocrinol. 212(2), 149–158 (2012). https://doi.org/10.1530/JOE-11-0288
L.F. Iannucci, F. Cioffi, R. Senese, F. Goglia, A. Lanni, P.M. Yen, R.A. Sinha, Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci. Rep. 7(1), 2023 (2017). https://doi.org/10.1038/s41598-017-02205-1
P. Fallahi, S.M. Ferrari, E. Santini, S. Camastra, G. Frenzilli, M. Puccini, F. Goglia, A. Lanni, P. Marchetti, A. Antonelli, Both 3,5-diiodo-L-thyronine (T2) and T3 modulate glucose-induced insulin secretion. J. Biol. Regul. Homeost. Agents 31(2), 503–508 (2017)
A. Lombardi, R. Senese, R. De Matteis, R.A. Busiello, F. Cioffi, F. Goglia, A. Lanni, 3,5-Diiodo-L-thyronine activates brown adipose tissue thermogenesis in hypothyroid rats. PLoS ONE 10(2), e0116498 (2015). https://doi.org/10.1371/journal.pone.0116498
R. Senese, F. Cioffi, R. De Matteis, G. Petito, P. de Lange, E. Silvestri, A. Lombardi, M. Moreno, F. Goglia, A. Lanni, 3,5 diiodo-l-thyronine (T(2)) promotes the browning of white adipose tissue in high-fat diet-induced overweight male rats housed at thermoneutrality. Cells 8(3) (2019). https://doi.org/10.3390/cells8030256
S. da Silva Teixeira, C. Filgueira, D.H. Sieglaff, C. Benod, R. Villagomez, L.J. Minze, A. Zhang, P. Webb, M.T. Nunes, 3,5-diiodothyronine (3,5-T2) reduces blood glucose independently of insulin sensitization in obese mice. Acta Physiol. (Oxf.) 220(2), 238–250 (2017). https://doi.org/10.1111/apha.12821
E. Silvestri, R. Senese, F. Cioffi, R. De Matteis, D. Lattanzi, A. Lombardi, A. Giacco, A.M. Salzano, A. Scaloni, M. Ceccarelli, M. Moreno, F. Goglia, A. Lanni, P. de Lange, 3,5-diiodo-l-thyronine exerts metabolically favorable effects on visceral adipose tissue of rats receiving a high-fat diet. Nutrients 11(2) (2019). https://doi.org/10.3390/nu11020278
E. Silvestri, F. Cioffi, R. De Matteis, R. Senese, P. de Lange, M. Coppola, A.M. Salzano, A. Scaloni, M. Ceccarelli, F. Goglia, A. Lanni, M. Moreno, A. Lombardi, 3,5-diiodo-l-thyronine affects structural and metabolic features of skeletal muscle mitochondria in high-fat-diet fed rats producing a co-adaptation to the glycolytic fiber phenotype. Front Physiol. 9, 194 (2018). https://doi.org/10.3389/fphys.2018.00194
A. Lombardi, A. Lanni, M. Moreno, M.D. Brand, F. Goglia, Effect of 3,5-di-iodo-L-thyronine on the mitochondrial energy-transduction apparatus. Biochem J. 330(Pt 1), 521–526 (1998). https://doi.org/10.1042/bj3300521
A. Cavallo, F. Taurino, F. Damiano, L. Siculella, A.M. Sardanelli, A. Gnoni, Acute administration of 3,5-diiodo-L-thyronine to hypothyroid rats stimulates bioenergetic parameters in liver mitochondria. J. Bioenerg. Biomembr. 48(5), 521–529 (2016). https://doi.org/10.1007/s10863-016-9686-4
A. Cavallo, A. Gnoni, E. Conte, L. Siculella, F. Zanotti, S. Papa, G.V. Gnoni, 3,5-diiodo-L-thyronine increases FoF1-ATP synthase activity and cardiolipin level in liver mitochondria of hypothyroid rats. J. Bioenerg. Biomembr. 43(4), 349–357 (2011). https://doi.org/10.1007/s10863-011-9366-3
A. Lombardi, P. de Lange, E. Silvestri, R.A. Busiello, A. Lanni, F. Goglia, M. Moreno, 3,5-Diiodo-L-thyronine rapidly enhances mitochondrial fatty acid oxidation rate and thermogenesis in rat skeletal muscle: AMP-activated protein kinase involvement. Am. J. Physiol. Endocrinol. Metab. 296(3), E497–E502 (2009). https://doi.org/10.1152/ajpendo.90642.2008
F. Cioffi, R. Senese, G. Petito, P. Lasala, P. de Lange, E. Silvestri, A. Lombardi, M. Moreno, F. Goglia, A. Lanni, Both 3,3′,5-triiodothyronine and 3,5-diodo-L-thyronine are able to repair mitochondrial DNA damage but by different mechanisms. Front Endocrinol. (Lausanne) 10, 216 (2019). https://doi.org/10.3389/fendo.2019.00216
A. Antonelli, P. Fallahi, S.M. Ferrari, A. Di Domenicantonio, M. Moreno, A. Lanni, F. Goglia, 3,5-diiodo-L-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J. Biol. Regul. Homeost. Agents 25(4), 655–660 (2011)
W.J. Wood, T. Geraci, A. Nilsen, A.E. DeBarber, T.S. Scanlan, Iodothyronamines are oxidatively deaminated to iodothyroacetic acids in vivo. Chembiochem 10(2), 361–365 (2009). https://doi.org/10.1002/cbic.200800607
S. Groeneweg, R.P. Peeters, T.J. Visser, W.E. Visser, Triiodothyroacetic acid in health and disease. J. Endocrinol. 234(2), R99–R121 (2017). https://doi.org/10.1530/JOE-17-0113
S.I. Sherman, P.W. Ladenson, Octreotide therapy of growth hormone excess in the McCune-Albright syndrome. J. Endocrinol. Invest 15(3), 185–190 (1992). https://doi.org/10.1007/BF03348702
S.I. Sherman, M.D. Ringel, M.J. Smith, H.A. Kopelen, W.A. Zoghbi, P.W. Ladenson, Augmented hepatic and skeletal thyromimetic effects of tiratricol in comparison with levothyroxine. J. Clin. Endocrinol. Metab. 82(7), 2153–2158 (1997). https://doi.org/10.1210/jcem.82.7.4054
S. Kersseboom, S. Horn, W.E. Visser, J. Chen, E.C. Friesema, C. Vaurs-Barriere, R.P. Peeters, H. Heuer, T.J. Visser, In vitro and mouse studies supporting therapeutic utility of triiodothyroacetic acid in MCT8 deficiency. Mol. Endocrinol. 28(12), 1961–1970 (2014). https://doi.org/10.1210/me.2014-1135
J. Delbaere, P. Vancamp, S.L. Van Herck, N.M. Bourgeois, M.J. Green, R.J. Wingate, V.M. Darras, MCT8 deficiency in Purkinje cells disrupts embryonic chicken cerebellar development. J. Endocrinol. 232(2), 259–272 (2017). https://doi.org/10.1530/JOE-16-0323
D. Zada, A. Tovin, T. Lerer-Goldshtein, L. Appelbaum, Pharmacological treatment and BBB-targeted genetic therapy for MCT8-dependent hypomyelination in zebrafish. Dis. Model Mech. 9(11), 1339–1348 (2016). https://doi.org/10.1242/dmm.027227
J.M. Kunitake, N. Hartman, L.C. Henson, J. Lieberman, D.E. Williams, M. Wong, J.M. Hershman, 3,5,3′-triiodothyroacetic acid therapy for thyroid hormone resistance. J. Clin. Endocrinol. Metab. 69(2), 461–466 (1989). https://doi.org/10.1210/jcem-69-2-461
D. Harrus, H. Demene, E. Vasquez, A. Boulahtouf, P. Germain, A.C. Figueira, M.L. Privalsky, W. Bourguet, A. le Maire, Pathological interactions between mutant thyroid hormone receptors and corepressors and their modulation by a thyroid hormone analogue with therapeutic potential. Thyroid 28(12), 1708–1722 (2018). https://doi.org/10.1089/thy.2017.0551
S. Horn, S. Kersseboom, S. Mayerl, J. Muller, C. Groba, M. Trajkovic-Arsic, T. Ackermann, T.J. Visser, H. Heuer, Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter mct8. Endocrinology 154(2), 968–979 (2013). https://doi.org/10.1210/en.2012-1628
S. Groeneweg, R.P. Peeters, T.J. Visser, W.E. Visser, Therapeutic applications of thyroid hormone analogues in resistance to thyroid hormone (RTH) syndromes. Mol. Cell Endocrinol. 458, 82–90 (2017). https://doi.org/10.1016/j.mce.2017.02.029
Y.T. Chin, Z.R. He, C.L. Chen, H.C. Chu, Y. Ho, P.Y. Su, Y.S.H. Yang, K. Wang, Y.J. Shih, Y.R. Chen, J.Z. Pedersen, S. Incerpi, A.W. Nana, H.Y. Tang, H.Y. Lin, S.A. Mousa, P.J. Davis, J. Whang-Peng, Corrigendum: tetrac and NDAT Induce Anti-proliferation via Integrin alphavbeta3 in Colorectal Cancers With Different K-RAS Status. Front Endocrinol. (Lausanne) 10, 241 (2019). https://doi.org/10.3389/fendo.2019.00241
P.J. Davis, H.Y. Tang, A. Hercbergs, H.Y. Lin, K.A. Keating, S.A. Mousa, Bioactivity of thyroid hormone analogs at cancer cells. Front Endocrinol. (Lausanne) 9, 739 (2018). https://doi.org/10.3389/fendo.2018.00739
G. Medina-Gomez, R.M. Calvo, M.J. Obregon, T3 and Triac inhibit leptin secretion and expression in brown and white rat adipocytes. Biochim Biophys. Acta 1682(1–3), 38–47 (2004). https://doi.org/10.1016/j.bbalip.2004.01.007
H.C. Ha, J.M. Jang, D. Zhou, H.G. Kim, M.J. Back, I.C. Shin, S.Y. Yun, Y. Piao, J.M. Choi, J.H. Won, D.K. Kim, 3, 5, 3′-Triiodothyroacetic acid (TRIAC) is an anti-inflammatory drug that targets toll-like receptor 2. Arch. Pharm. Res 41(10), 995–1008 (2018). https://doi.org/10.1007/s12272-018-1057-8
J.L. Leonard, Non-genomic actions of thyroid hormone in brain development. Steroids 73(9–10), 1008–1012 (2008). https://doi.org/10.1016/j.steroids.2007.12.016
J.T. Domingues, D. Cattani, P.A. Cesconetto, B.A. Nascimento de Almeida, P. Pierozan, K. Dos Santos, G. Razzera, F.R. Mena Barreto Silva, R. Pessoa-Pureur, A. Zamoner, Reverse T3 interacts with alphavbeta3 integrin receptor and restores enzyme activities in the hippocampus of hypothyroid developing rats: Insight on signaling mechanisms. Mol. Cell Endocrinol. 470, 281–294 (2018). https://doi.org/10.1016/j.mce.2017.11.013
H.Y. Lin, H.Y. Tang, M. Leinung, S.A. Mousa, A. Hercbergs, P.J. Davis, Action of Reverse T3 on Cancer Cells. Endocr Res, 1–5 (2019). https://doi.org/10.1080/07435800.2019.1600536
F. Economidou, E. Douka, M. Tzanela, S. Nanas, A. Kotanidou, Thyroid function during critical illness. Horm. (Athens) 10(2), 117–124 (2011). https://doi.org/10.14310/horm.2002.1301
L. Rastogi, M.M. Godbole, R.A. Sinha, S. Pradhan, Reverse triiodothyronine (rT3) attenuates ischemia-reperfusion injury. Biochem Biophys. Res Commun. 506(3), 597–603 (2018). https://doi.org/10.1016/j.bbrc.2018.10.031
Acknowledgements
This research was financially supported by a “FAR” grant from the University of Sannio and was supported by University of Campania “Luigi Vanvitelli” Programma VALERE: Vanvitelli per la Ricerca.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Senese, R., Cioffi, F., Petito, G. et al. Thyroid hormone metabolites and analogues. Endocrine 66, 105–114 (2019). https://doi.org/10.1007/s12020-019-02025-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02025-5