Abstract
Pourposes
We investigated the expression of RET9 and RET51 isoforms in medullary (MTC), papillary (PTC) thyroid carcinoma, normal thyroid tissues, and pheochromocytoma (PHEO) to verify if these isoforms are present also in follicular thyroid cell-derived tissues, and if there is a differential expression of RET9 and RET51 in MTC.
Methods
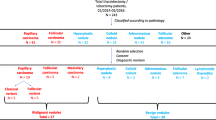
Nineteen patients with MTC, 18 patients with PTC, 18 samples of contralateral normal thyroid tissues, and 5 cases of PHEO were included in this study. RET isoform expression was studied by real-time RT-PCR.
Results
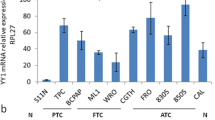
All MTCs and PHEOs were positive for RET9 and RET51. Fourteen/eighteen (77.7%) PTC cases were positive for RET9 and/or RET51, and four were positive for only one of the genes. In normal thyroid tissues, 3/18 (16.7%) cases were negative for both isoforms, 4/18 (22.2%) were positive for both, and 11/18 (61.1%) were positive for only one. RET isoforms were expressed at different levels in MTC, PHEO, PTC, and normal thyroid tissues: RET9 expression was higher in PHEO than in MTC, PTC, and normal thyroid tissues. RET9 expression was also higher in MTC than in PTC and normal thyroid tissues. No difference was observed between PTC and normal thyroid tissues. A similar pattern of expression was observed for RET51. In addition, RET51 was significantly more expressed than RET9 in MTC, while RET9 was the predominant isoform in PHEO.
Conclusions
Our study documented the expression of the RET9 and RET51 isoforms in normal thyroid and PTC tissues. RET9 and RET51 isoforms were also present in MTC and PHEO. RET51 expression was higher than RET9 expression in MTC, while there was no difference in the expression of these two isoforms in PTC and normal thyroid tissues. RET9 was more highly expressed than RET51 in PHEOs.







Similar content being viewed by others
References
P. Durbec, C.V. Marcos-Gutierrez, C. Kilkenny, M. Grigoriou, K. Wartiowaara, P. Suvanto, D. Smith, B. Ponder, F. Costantini, M. Saarma, GDNF signalling through the Ret receptor tyrosine kinase. Nature 381, 789 (1996)
R.H. Baloh, H. Enomoto, E.M.J. Johnson, J. Milbrandt, The GDNF family ligands and receptors - implications for neural development. Curr. Opin. Neurobiol. 10, 103 (2000)
E. Arighi, M.G. Borrello, H. Sariola, RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 16, 441 (2005)
K. Takaya, T. Yoshimasa, H. Arai, N. Tamura, Y. Miyamoto, H. Itoh, K. Nakao, Expression of the RET proto-oncogene in normal human tissues, pheochromocytomas, and other tumors of neural crest origin. J. Mol. Med (Berl.) 74, 617 (1996)
P. Kjellman, D.L. Learoyd, M. Messina, G. Weber, A. Hoog, G. Wallin, C. Larsson, B.G. Robinson, J. Zedenius, Expression of the RET proto-oncogene in papillary thyroid carcinoma and its correlation with clinical outcome. Br. J. Surg. 88, 557 (2001)
M. Inaba, S. Umemura, H. Satoh, Y. Abe, K. Kurokawa, H. Sakai, R.Y. Osamura, Expression of RET in follicular cell-derived tumors of the thyroid gland: prevalence and implication of morphological type. Pathol. Int. 53, 146 (2003)
O. Fluge, D.R. Haugen, L.A. Akslen, A. Marstad, M. Santoro, A. Fusco, J.E. Varhaug, J.R. Lillehaug, Oncogene 20, 885 (2001)
S.M. Ivanchuk, S.M. Myers, L.M. Mulligan, Expression and alternative splicing of c-ret RNA in papillary thyroid carcinomas. Oncogene 16, 991 (1998)
T. Tahira, Y. Ishizaka, F. Itoh, T. Sugimura, M. Nagao, Tahira, Y. Ishizaka, F. Itoh, T. Sugimura, M. Nagao, Expression of the ret proto-oncogene in human neuroblastoma cell lines and its increase during neuronal differentiation induced by retinoic acid. Oncogene 5, 97 (1990)
S.M. Myers, C. Eng, B.A. Ponder, L.M. Mulligan, Characterization of RET proto-oncogene 3' splicing variants and polyadenylation sites: a novel C-terminus for RET. Oncogene 11, 2039 (1995)
H. Le Hir, N. Charlet-Berguerand, A. Gimenez-Roqueplo, M. Mannelli, P. Plouin, V. de Franciscis, C. Thermes, Relative expression of the RET9 and RET51 isoforms in human pheochromocytomas. Oncology 58, 311 (2000)
D.S. Richardson, D.M. Rodrigues, B.D. Hyndman, M.J.F. Crupi, A.C. Nicolescu, L.M. Mulligan, Alternative splicing results in RET isoforms with distinct trafficking properties. Mol. Biol. Cell. 23, 3838 (2012)
E.Y. Lian, S.M. Maritan, J.G. Cockburn, K. Kasaian, M.J.F. Crupi, D. Hurlbut, S.J.M. Jones, S.M. Wiseman, L.M. Mulligan, Differential roles of RET isoforms in medullary and papillary thyroid carcinomas. Endocr. Relat. Cancer 24, 53 (2017)
C. Romei, R. Ciampi, R. Elisei, A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat. Rev. Endocrinol. 12, 192 (2016)
G. Bunone, M. Uggeri, P. Mondellini, M.A. Pierotti, I. Bongarzone, RET receptor expression in thyroid follicular epithelial cell-derived tumors. Cancer Res. 60, 2845 (2000)
M.T. Carter, J.L. Yome, M.N. Marcil, C.A. Martin, J.B. Vanhorne, L.M. Mulligan, Conservation of RET proto-oncogene splicing variants and implications for RET isoform function. Cytogenet. Cell Genet. 95, 169 (2001)
M. Rossel, A. Pasini, S. Chappuis, O. Geneste, L. Fournier, I. Schuffenecker, M. Takahashi, L.A. van Grunsven, J.L. Urdiales, B.B. Rudkin, G.M. Lenoir, M. Billaud, Distinct biological properties of two RET isoforms activated by MEN 2A and MEN 2B mutations. Oncogene 14, 265 (1997)
T. Iwashita, M. Kato, H. Murakami, N. Asai, Y. Ishiguro, S. Ito, Y. Iwata, K. Kawai, M. Asai, K. Kurokawa, H. Kajita, M. Takahashi, Biological and biochemical properties of Ret with kinase domain mutations identified in multiple endocrine neoplasia type 2B and familial medullary thyroid carcinoma. Oncogene 18, 3919 (1999)
J. Turchini, V.K.Y. Cheung, A.S. Tischler, R.R. De Krijger, A.J. Gill, Pathology and genetics of phaeochromocytoma and paraganglioma. Histopathology 72, 97 (2018)
C. Guerin, P. Romanet, D. Taieb, T. Brue, A. Lacroix, F. Sebag, A. Barlier, F. Castinetti, Looking beyond the thyroid: advances in the understanding of pheochromocytoma and hyperparathyroidism phenotypes in MEN2 and of non-MEN2 familial forms. Endocr. Relat. Cancer 25, T15 (2018)
Acknowledgements
This study has been supported by Associazione Italiana Ricerca sul Cancro (IG 2018, cod 21790) and Agenzia Italiana del Farmaco (Cod AIFA-2016-02365049).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest that could affect the impartiality of the reported research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramone, T., Romei, C., Ciampi, R. et al. Differential expression of RET isoforms in normal thyroid tissues, papillary and medullary thyroid carcinomas. Endocrine 65, 623–629 (2019). https://doi.org/10.1007/s12020-019-01957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01957-2