Abstract
Purpose
In obesity, metabolic and voluntary factors regulate appetite, and a dysregulation of the reward pathway was demonstrated in all addiction disorders. Deep transcranial magnetic stimulation (dTMS) is already used to modulate cerebral dopamine activation in neuro-psychiatric diseases. We presently assess the acute effect of high frequency (HF) and low frequency (LF) dTMS on the modulation of the main neuropeptides and neurotransmitters involved in the reward pathway in obese subjects.
Methods
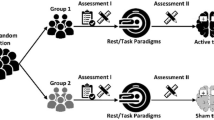
This study was designed as a double-blind, sham-controlled, randomized clinical trial. Thirty-three obese patients (9 males, 24 females, age 48.1 ± 10.6, BMI 36.4 ± 4.7) were enrolled in the study. All patients were studied during a single dTMS session and blood aliquots were drawn before and after a single dTMS session. Metabolic and neuro-endocrine parameters were evaluated before and after: (1) 18 Hz dTMS (HF, 13 patients); (2) 1 Hz dTMS (LF, 10 patients); (3) Sham treatment (Sham, 10 patients).
Results
No statistically significant variations in metabolic parameters, systolic and diastolic blood pressure, and heart rate were shown acutely. HF showed a significant increase of β-endorphin compared to other groups (p = 0.048); a significant increase of ghrelin in LF (p = 0.041) was also demonstrated.
Conclusions
A single session of HF dTMS treatment determines in obese subjects an acute increase of β-endorphin level, indicating an activation of the reward pathway. The present findings constitute proof of principle for a potential application of this methodology in obesity treatment.




Similar content being viewed by others
References
T.A. Hare, C.F. Camerer, A. Rangel, Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324(5927), 646–648 (2009)
M.E. Gluck, P. Viswanath, E.J. Stinson, Obesity, appetite, and the prefrontal cortex. Curr. Obes. Rep. 6(4), 380–388 (2017)
J. McClelland, N. Bozhilova, I. Campbell, U. Schmidt, A systematic review of the effects of neuromodulation on eating and body weight: evidence from human and animal studies. Eur. Eat. Disord. Rev. 21(6), 436–455 (2013)
D. Val-Laillet, E. Aarts, B. Weber, M. Ferrari, V. Quaresima, L.E. Stoeckel, M. Alonso-Alonso, M. Audette, C.H. Malbert, E. Stice, Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 8, 1–31 (2015)
N.D. Volkow, G.J. Wang, F. Telang, J.S. Fowler, P.K. Thanos, J. Logan, D. Alexoff, Y.S. Ding, C. Wong, Y. Ma, K. Pradhan, Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42(4), 1537–1543 (2008)
D.M. Small, R.J. Zatorre, A. Dagher, A.C. Evans, M. Jones-Gotman, Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124, 1720–1733 (2001)
G.J. Wang, N.D. Volkow, J.S. Fowler, The role of dopamine in motivation for food in humans: implications for obesity. Expert. Opin. Ther. Targets 6(5), 601–609 (2002)
J.H. Baik, Dopamine signaling in food addiction: role of dopamine D2 receptors. Bmb. Rep. 46(11), 519–526 (2013)
E. Hadley, C. Haskell-Luevano, The Propiomelanocortin System. Ann. N. Y. Acad. Sci. 885, 1–21 (1999)
B.C. Field, Neuroendocrinology of obesity. Br. Med. Bull. 109, 73–82 (2014)
M. Bose, B. Oliván, B. Laferrère, Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr. Opin. Endocrinol. Diabetes Obes. 16(5), 340–346 (2009)
G.S. Pell, Y. Roth, A. Zangen, Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 93(1), 59–98 (2011)
E.M. Wassermann, S.H. Lisanby, Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin. Neurophysiol. 112(8), 1367–1377 (2001)
A. Zangen, Y. Roth, B. Voller, M. Hallett, Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin. Neurophysiol. 116(4), 775–779 (2005)
M. Grall-Bronnec, A. Sauvaget, The use of repetitive transcranial magnetic stimulation for modulating craving and addictive behaviours: a critical literature review of efficacy, technical and methodological considerations. Neurosci. Biobehav. Rev. 47, 592–613 (2014)
G. Addolorato, L. Leggio, F.W. Hopf, M. Diana, A. Bonci, Novel therapeutic strategies for alcohol and drug addiction: focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology 37(1), 163–177 (2012)
B.R. Mishra, S.H. Nizamie, B. Das, S.K. Praharaj, Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105(1), 49–55 (2010)
G. Addolorato, M. Antonelli, F. Cocciolillo, G.A. Vassallo, C. Tarli, L. Sestito, A. Mirijello, A. Ferrulli, D.A. Pizzuto, G. Camardese, A. Miceli, M. Diana, A. Giordano, A. Gasbarrini, D. Di Giuda, Deep transcranial magnetic stimulation of the dorsolateral prefrontal cortex in alcohol use disorder patients: effects on dopamine transporter availability and alcohol intake. Eur. Neuropsychopharmacol. 27(5), 450–461 (2017)
L. Dinur-Klein, P. Dannon, A. Hadar, O. Rosenberg, Y. Roth, M. Kotler, A. Zangen, Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76(9), 742–749 (2014)
A. Terraneo, L. Leggio, M. Saladini, M. Ermani, A. Bonci, L. Gallimberti, Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur. Neuropsychopharmacol. 26(1), 37–44 (2016)
M. Kanda, T. Mima, T. Oga, M. Matsuhashi, K. Toma, H. Hara, T. Satow, T. Nagamine, J.C. Rothwell, H. Shibasaki, Transcranial magnetic stimulation (TMS) of the sensorimotor cortex and medial frontal cortex modifies human pain perception. Clin. Neurophysiol. 114, 160–166 (2003)
C. Rapinesi, A. Del Casale, P. Scatena, G.D. Kotzalidis, S. Di Pietro, V.R. Ferri, F.S. Bersani, R. Brugnoli, R.N. Raccah, A. Zangen, S. Ferracuti, F. Orzi, P. Girardi, G. Sette, Add-on deep Transcranial Magnetic Stimulation (dTMS) for the treatment of chronic migraine: a preliminary study. Neurosci. Lett. 623, 7–12 (2016)
E. Yates, G. Balu, Deep Transcranial Magnetic Stimulation: A Promising Drug-Free Treatment Modality in the Treatment of Chronic Low Back Pain. Del. Med. J. 88(3), 90–92 (2016)
M.B. First, R.L. Spitzer, M. Gibbon, J.B. Williams,: Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP). New York, New York, USA: Biometrics Research, New York State Psychiatric Institute (2002)
K.C. Berridge, The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191(3), 391–431 (2007)
A.P. Strafella, T. Paus, J. Barret, A. Dagher, Repetitive transcranical magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 21(15), RC157 (2001)
M.F. Maranhão, N.M. Estella, M.E. Cury, V.L. Amigo, C.M. Picasso, A. Berberian, I. Campbell, U. Schmidt, A.M. Claudino, The effects of repetitive transcranial magnetic stimulation in obese females with binge eating disorder: a protocol for a double-blinded, randomized, sham-controlled trial. BMC Psychiatry 15, 194 (2015)
K. Fujioka, Sustained-release naltrexone/bupropion-a novel pharmacologic approach to obesity and food craving. US Endocrinol. 10, 53–58 (2014)
C. Gianoulakis, Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J. Psychiatry Neurosci. 26(4), 304–318 (2001)
J.J. Tuulari, L. Tuominen, F.E. de Boer, J. Hirvonen, S. Helin, P. Nuutila, L. Nummenmaa, Feeding releases endogenous opioids in humans. J. Neurosci. 37(34), 8284–8291 (2017)
K. Blum, E.R. Braverman, J.M. Holder, J.F. Lubar, V.J. Monastra, D. Miller, J.O. Lubar, T.J. Chen, D.E. Comings, Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs 32(Suppl:i-iv), 1–112 (2000)
M.A. Ahmed, S.A. Mohamed, D. Sayed, Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol. Res. 33(9), 953–958 (2011)
X. Li, R.J. Malcolm, K. Huebner, C.A. Hanlon, J.J. Taylor, K.T. Brady, M.S. George, R.E. See, Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: a preliminary study. Drug Alcohol. Depend. 133(2), 641–646 (2013)
Y. Dikshtein, R. Barnea, N. Kronfeld, E. Lax, I. Roth-Deri, A. Friedman, I. Gispan, E. Elharrar, S. Levy, M. Ben-Tzion, G. Yadid, β-endorphin via the Delta Opioid receptor is a major factor in the incubation of the cocaine cravings. Neuropsychopharmacology 38, 2508–2514 (2013)
T. Ledoux, A.S. Nguyen, C. Bakos-Block, P. Bordnick, Using virtual reality to study food cravings. Appetite 71, 396–402 (2013)
Acknowledgements
This study was supported by the Italian Ministry of Health (RF-2011–02349303).
Funding
Prof. Livio Luzi was a recipient of a grant from the Italian Ministry of Health (RF-2011–02349303).
Author information
Authors and Affiliations
Contributions
LL and IT contributed to designing the research study. LL, AF, and CM conducted experiments; specifically, LL provided research conduct oversight; AF contributed to performing dTMS after a specific training, and to providing medical oversight; CM contributed to collecting blood samples. AF and MA contributed to acquiring data; FA and VM performed statistical analysis. AF, LL, MA, FA and VM contributed to writing the manuscript. As corresponding author, LL confirms that he had full access to all the data in the study and has final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ferrulli, A., Macrì, C., Terruzzi, I. et al. High frequency deep transcranial magnetic stimulation acutely increases β-endorphins in obese humans. Endocrine 64, 67–74 (2019). https://doi.org/10.1007/s12020-018-1791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1791-1