Abstract
Purpose
somatic mutations in the ubiquitin-specific protease 8 (USP8) gene have recently been described in patients with Cushing’s disease (CD). The aim of the study is to verify whether USP8 mutation may predict early and late outcome of pituitary surgery in patients with CD operated at a single institution.
Methods
We performed a retrospective genetic analysis of 92 adrenocorticotropic hormone (ACTH)-secreting pituitary adenomas. Specimens were screened for USP8 hotspot mutations in the exon 14 with Sanger sequencing. Hormonal and surgical data were compared between USP8 variant carriers and wild-type tumors.
Results
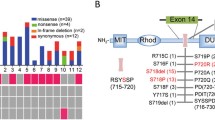
USP8 variants were detected in 22 adenomas (23.9%) with higher prevalence in women (28.9% vs. 5.3% in men; p < 0.05). No significant difference in hormonal levels and tumoral features in relation to USP8 status was observed. Interestingly, USP8-variant carriers were more likely to achieve surgical remission than wild-type adenomas (100% vs. 75.7%; p = 0.01). Conversely, recurrence of CD occurred in 23% of USP8-mutated patients and in 13% of patients with wild-type adenoma. The recurrence-free survival did not differ significantly between the two groups (p = 0.42).
Conclusions
ACTH-secreting pituitary adenomas carrying somatic USP8 mutations are associated with a greater likelihood of surgical remission in patients operated by a single neurosurgeon. Recurrence rates are not related with USP8-variant status.

Similar content being viewed by others
References
O.M. Dekkers, E. Horvath-Puho, J.O. Jørgensen, S.C. Cannegieter, V. Ehrenstein, J.P. Vandenbroucke et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J. Clin. Endocrinol. Metab. 98, 2277–2284 (2013)
L.K. Nieman, B.M. Biller, J.W. Findling, M.H. Murad, J. Newell-Price, M.O. Savage et al. Treatment of Cushing’s syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 2807–2831 (2015)
D. Bochicchio, M. Losa, M. Buchfelder, Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J. Clin. Endocrinol. Metab. 80, 3114–3120 (1995)
B.M. Hofman, M. Hlavac, R. Martinez, M. Buchfelder, O.A. Muller, R. Fahlbusch, Long-term results after microsurgery for Cushing’s disease: experience with 426 primary operations over 35 years. J. Neurosurg. 108, 9–18 (2008)
W.F. Chandler, A.L. Barkan, T. Hollon, A. Sakharova, J. Sack, B. Brahma et al. Outcome of transsphenoidal surgery for Cushing disease: a single–center experience over 32 years. Neurosurgery 78, 216–223 (2016)
R. Pivonello, M. De Leo, A. Cozzolino, A. Colao, The treatment of Cushing’s disease. Endocr. Rev. 36, 385–486 (2015)
M. Losa, R. Bianchi, R. Barzaghi, M. Giovanelli, P. Mortini, Persistent ACTH response to desmopressin in the early postoperative period is a risk factor for recurrence of Cushing’s disease. J. Clin. Endocrinol. Metab. 94, 3322–3328 (2009)
S. Melmed, Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 7, 257–266 (2011)
S. Sbiera, T. Deutschbein, I. Weigand, M. Reincke, M. Fassnacht, B. Allolio, The new molecular landscape of Cushing’s disease. Trends Endocrinol. Metab. 26, 573–583 (2015)
C.A. Stratakis, Diagnosis and clinical genetics of Cushing syndrome in pediatrics. Endocrinol. Metab. Clin. North. Am. 45, 311–328 (2016)
A. Albani, M. Theodoropoulou, M. Reincke, Genetics of Cushing’s disease. Clin. Endocrinol. (Oxf.). 88, 3–12 (2018)
Z.Y. Ma, Z.J. Song, J.H. Chen, Y.F. Wang, S.Q. Li, L.F. Zhou et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 25, 306–317 (2015)
M. Reincke, S. Sbiera, A. Hayakawa, M. Theodoropoulou, A. Osswald, F. Beuschlein et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 47, 31–38 (2015)
L.G. Pérez-Rivas, M. Theodoropoulou, F. Ferraù, C. Nusser, K. Kawaguchi, C.A. Stratakis et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J. Clin. Endocrinol. Metab. 100, E997–E1004 (2015)
K. Hayashi, N. Inoshita, K. Kawaguchi, A. Ibrahim Ardisasmita, H. Suzuki, N. Fukuhara et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur. J. Endocrinol. 174, 213–226 (2016)
F.R. Faucz, A. Tirosh, C. Tatsi, A. Berthon, L.C. Hernandez-Ramirez, N. Settas et al. Somatic USP8 gene mutations are a common cause of pediatric Cushing disease. J. Clin. Endocrinol. Metab. 102, 2836–2843 (2017)
M. Theodoropoulou, T. Arzberger, Y. Gruebler, M.L. Jaffrain-Rea, J. Schlegel, L. Schaaf et al. Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J. Endocrinol. 183, 385–394 (2004)
H. Fukuoka, O. Cooper, A. Ben-Shlomo, A. Mamelak, S.G. Ren, D. Bruyette et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Invest. 121, 4712–4721 (2011)
M. Losa, P. Mortini, S. Dylgjeri, R. Barzaghi, A. Franzin, C. Mandelli et al. Desmopressin stimulation test before and after pituitary surgery in patients with Cushing’s disease. Clin. Endocrinol. (Oxf.). 55, 61–68 (2001)
M.F. Cassarino, A.G. Ambrogio, A. Cassarino, M.R. Terreni, D. Gentilini, A. Sesta et al. Gene expression profiling in human corticotrope tumors reveals distinct, neuroendocrine profiles. J. Neuroendocrinol. 30, e12628 (2018)
M.F. Cassarino, A. Sesta, L. Pagliardini, M. Losa, G. Lasio, F. Cavagnini et al. Proopiomelanocortin, glucocorticoid, and CRH receptor expression in human ACTH-secreting pituitary adenomas. Endocrine 55, 853–860 (2017)
F. Pecori Giraldi, L. Pagliardini, M.F. Cassarino, M. Losa, G. Lasio, F. Cavagnini, Responses to corticotrophin-releasing hormone and dexamethasone in a large series of human adrenocorticotrophic hormone-secreting pituitary adenomas in vitro reveal manifold corticotroph tumoural phenotypes. J. Neuroendocrinol. 23, 1214–1221 (2011)
M. Ravo, M. Mutarelli, L. Ferraro, O.M. Grober, O. Paris, R. Tarallo et al. Quantitative expression profiling of highly degraded RNA from formalin-fixed, paraffin-embedded breast tumor biopsies by oligonucleotide microarrays. Lab. Invest. 88, 430–440 (2008)
C. Ballmann, A. Thiel, H.E. Korah, A.C. Reis, W. Saeger, S. Stepanow et al. USP8 mutations in pituitary Cushing adenomas – targeted analysis by next-generation sequencing. J. Endocr. Soc. 2, 266–278 (2018)
L.G. Pérez-Rivas, M. Theodoropoulou, T.H. Puar, J. Fazel, M.R. Stieg, F. Ferraù et al. Somatic USP8 mutations are frequent events in corticotroph tumor progression causing Nelson’s tumor. Eur. J. Endocrinol. 178, 59–65 (2018)
A., Albani, L.G., Pérez-Rivas, C., Dimopoulou, S., Zopp, P., Colon-Bolea, S., Roeber, et al. The USP8 mutational status may predict long-term remission in patients with Cushing’s disease. Clin. Endocrinol. (Oxf). (2018). https://doi.org/10.1111/cen.13802
S.S. Chaidarun, B. Swearingen, J.M. Alexander, Differential expression of estrogen receptor-ß (ER ß) in human pituitary tumors: functional interactions with ER α and a tumor-specific splice variant. J. Clin. Endocrinol. Metab. 83, 3308–3315 (1998)
S. Oomizu, J. Honda, S. Takeuchi, T. Kakeya, T. Masui, S. Takahashi, Transforming growth factor-α stimulates proliferation of mammotrophs and corticotrophs in the mouse pituitary. J. Endocrinol. 165, 493–501 (2000)
F.F. Casanueva, A.L. Barkan, M. Buchfelder, A. Klibanski, E.R. Laws, J.S. Loeffler et al. Criteria for the definition of pituitary tumor centers of excellence (PTCOE): a Pituitary Society statement. Pituitary 20, 489–498 (2017)
Funding
This research did not receive any specific grant from any agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Losa, M., Mortini, P., Pagnano, A. et al. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine 63, 240–246 (2019). https://doi.org/10.1007/s12020-018-1776-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1776-0