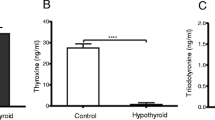
Abstract
Hypothyroidism and thyrotoxicosis produce adverse effects in male reproduction by unknown mechanisms. We investigated whether triiodothyronine (T3) modulates luteinizing hormone (LH) and follicle stimulating hormone (FSH) synthesis/secretion, by inducing different thyroid states. In hypothyroidism, the content of Lhb and Fshb mRNAs was increased, while their association to ribosomes and the protein content were reduced and the serum LH and FSH concentrations were augmented and decreased, respectively. Thyrotoxicosis reduced Lhb mRNA and LH serum concentration, and increased Lhb mRNA translational rate. The Fshb mRNA content and its association to ribosomes were also increased, whereas FSH serum concentrations were comparable to euthyroid levels. Acute T3 treatment decreased the total content of Lhb and Fshb mRNAs, and increased their association to ribosomes, as well as the LHB and FSHB contents in secretory granules. This study shows that T3 acts on gonadotrophs, resulting in direct effects on LH and FSH synthesis/secretion of male rats, suggesting that some reproductive disorders observed in men may be associated with thyroid hormone imbalances.









Similar content being viewed by others
References
P. Donnelly, C. White, Testicular dysfunction in men with primary hypothyroidism; reversal of hypogonadotrophic hypogonadism with replacement thyroxine. Clin. Endocrinol. 52(2), 197–201 (2000)
N. Tagawa, T. Takano, S. Fukata, K. Kuma, H. Tada, Y. Izumi, Y. Kobayashi, N. Amino, Serum concentration of androstenediol and androstenediol sulfate in patients with hyperthyroidism and hypothyroidism. Endocr. J. 48(3), 345–354 (2001)
A. Iranmanesh, G. Lizarralde, M.L. Johnson, J.D. Veldhuis, Dynamics of 24-hour endogenous cortisol secretion and clearance in primary hypothyroidism assessed before and after partial thyroid hormone replacement. J. Clin. Endocrinol. Metab. 70(1), 155–161 (1990). https://doi.org/10.1210/jcem-70-1-155
J. Nielsen, R.B. Jensen, A. Juul, Increased sex hormone-binding globulin levels in children and adolescents with thyrotoxicosis. Horm. Res. Paediatr. 79, 157–61 (2013). https://doi.org/10.1159/000348837
M. Pugeat, N. Nader, K. Hogeveen, G. Raverot, H. Dechaud, C. Grenot, Sex hormone-binding globulin gene expression in the liver: drugs and the metabolic syndrome. Mol. Cell. Endocrinol. 316(1), 53–59 (2010). https://doi.org/10.1016/j.mce.2009.09.020
M.R. Nikoobakht, M. Aloosh, N. Nikoobakht, A.R. Mehrsay, F. Biniaz, M.A. Karjalian, The role of hypothyroidism in male infertility and erectile dysfunction. Urol. J. 9(1), 405–409 (2012)
G.E. Krassas, K. Tziomalos, F. Papadopoulou, N. Pontikides, P. Perros, Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J. Clin. Endocrinol. Metab. 93(5), 1815–1819 (2008). https://doi.org/10.1210/jc.2007-2259
G.E. Krassas, F. Papadopoulou, K. Tziomalos, T. Zeginiadou, N. Pontikides, Hypothyroidism has an adverse effect on human spermatogenesis: a prospective, controlled study. Thyroid 18(12), 1255–1259 (2008). https://doi.org/10.1089/thy.2008.0257
G.E. Krassas, N. Pontikides, Male reproductive function in relation with thyroid alterations. Best Pract. Res. Clin. Endocrinol. Metab. 18(2), 183–195 (2004). https://doi.org/10.1016/j.beem.2004.03.003
S. Rojdmark, A. Berg, G. Kallner, Hypothalamic-pituitary-testicular axis in patients with hyperthyroidism. Horm. Res. 29(5–6), 185–190 (1988)
A.W. Meikle, The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid 14(3, Suppl. 1), 17–25 (2004)
S. La Vignera, R. Vita, R.A. Condorelli, L.M. Mongioì, S. Presti, S. Benvenga, A.E. Calogero, Impact of thyroid disease on testicular function. Endocrine 58(3), 397–407 (2017). https://doi.org/10.1007/s12020-017-1303-8
G.E. Krassas, K. Poppe, D. Glinoer, Thyroid function and human reproductive health. Endocr. Rev. 31(5), 702–755 (2010). https://doi.org/10.1210/er.2009-0041
E. Krajewska-Kulak, P. Sengupta, Thyroid function in male infertility. Front. Endocrinol. 4, 174 (2013). https://doi.org/10.3389/fendo.2013.00174
R.M. Romano, P. Bargi-Souza, E.L. Brunetto, F. Goulart-Silva, M.C. Avellar, C.A. Oliveira, M.T. Nunes, Hypothyroidism in adult male rats alters posttranscriptional mechanisms of luteinizing hormone biosynthesis. Thyroid 23(4), 497–505 (2013). https://doi.org/10.1089/thy.2011.0514
K. Czaplinski, R.H. Singer, Pathways for mRNA localization in the cytoplasm. Trends Biochem. Sci. 31(12), 687–693 (2006). https://doi.org/10.1016/j.tibs.2006.10.007
J. Hesketh, Translation and the cytoskeleton: a mechanism for targeted protein synthesis. Mol. Biol. Rep. 19(3), 233–243 (1994)
S. Kindler, H. Wang, D. Richter, H. Tiedge, RNA transport and local control of translation. Annu. Rev. Cell Dev. Biol. 21, 223–245 (2005). https://doi.org/10.1146/annurev.cellbio.21.122303.120653
Y. Funakoshi, Y. Doi, N. Hosoda, N. Uchida, M. Osawa, I. Shimada, M. Tsujimoto, T. Suzuki, T. Katada, S. Hoshino, Mechanism of mRNA deadenylation: evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes Dev. 21(23), 3135–3148 (2007). https://doi.org/10.1101/gad.1597707
J.L. Leonard, Non-genomic actions of thyroid hormone in brain development. Steroids 73(9–10), 1008–1012 (2008). https://doi.org/10.1016/j.steroids.2007.12.016
F.G. Silva, G. Giannocco, M.F. Santos, M.T. Nunes, Thyroid hormone induction of actin polymerization in somatotrophs of hypothyroid rats: potential repercussions in growth hormone synthesis and secretion. Endocrinology 147(12), 5777–5785 (2006). https://doi.org/10.1210/en.2006-0110
P. Bargi-Souza, R.M. Romano, M. Salgado Rde, F. Goulart-Silva, E.L. Brunetto, T.M. Zorn, M.T. Nunes, Triiodothyronine rapidly alters the TSH content and the secretory granules distribution in male rat thyrotrophs by a cytoskeleton rearrangement-independent mechanism. Endocrinology 154(12), 4908–4918 (2013). https://doi.org/10.1210/en.2013-1508
R. Regazzi. Molecular Mechanisms of Exocytosis. (Landes Bioscience and Springer Science, New York, 2007)
W.H. Dillmann, S. Berry, N.M. Alexander, A physiological dose of triiodothyronine normalizes cardiac myosin adenosine triphosphatase activity and changes myosin isoenzyme distribution in semistarved rats. Endocrinology 112(6), 2081–2087 (1983)
P. Chomczynski, N. Sacchi, Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162(1), 156–159 (1987). https://doi.org/10.1006/abio.1987.9999
M.A. Romano, R.M. Romano, L.D. Santos, P. Wisniewski, D.A. Campos, P.B. de Souza, P. Viau, M.M. Bernardi, M.T. Nunes, C.A. de Oliveira, Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch. Toxicol. 86(4), 663–673 (2012). https://doi.org/10.1007/s00204-011-0788-9
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4), 402–408 (2001). https://doi.org/10.1006/meth.2001.1262
M.W. Pfaffl, A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29(9), e45 (2001)
M.J. del Prete, R. Vernal, H. Dolznig, E.W. Mullner, J.A. Garcia-Sanz, Isolation of polysome-bound mRNA from solid tissues amenable for RT-PCR and profiling experiments. RNA 13(3), 414–421 (2007). https://doi.org/10.1261/rna.79407
P. Szkodziak, S. Wozniak, P. Czuczwar, E. Wozniakowska, P. Milart, A. Mroczkowski, T. Paszkowski, Infertility in the light of new scientific reports—focus on male factor. Ann. Agric. Environ. Med. 23(2), 227–230 (2016). https://doi.org/10.5604/12321966.1203881
P. Patrizio, F. Sanguineti, D. Sakkas, Modern andrology: from semen analysis to postgenomic studies of the male gametes. Ann. N. Y. Acad. Sci. 1127, 59–63 (2008). https://doi.org/10.1196/annals.1434.021
M. Punab, O. Poolamets, P. Paju, V. Vihljajev, K. Pomm, R. Ladva, P. Korrovits, M. Laan, Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod. 32(1), 18–31 (2017). https://doi.org/10.1093/humrep/dew284
C. Krausz, Male infertility: pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 25(2), 271–285 (2011). https://doi.org/10.1016/j.beem.2010.08.006
F. Lotti, E. Maseroli, N. Fralassi, S. Degl’Innocenti, L. Boni, E. Baldi, M. Maggi, Is thyroid hormones evaluation of clinical value in the work-up of males of infertile couples? Hum. Reprod. 31(3), 518–529 (2016). https://doi.org/10.1093/humrep/dev338
R.M. Romano, S.N. Gomes, N.C. Cardoso, L. Schiessl, M.A. Romano, C.A. Oliveira, New insights for male infertility revealed by alterations in spermatic function and differential testicular expression of thyroid-related genes. Endocrine 55(2), 607–617 (2017). https://doi.org/10.1007/s12020-016-0952-3
U.B. Kaiser, A. Jakubowiak, A. Steinberger, W.W. Chin, Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro 1. Endocrinology 138(3), 1224–31 (1997)
L. Nagirnaja, K. Rull, L. Uusküla, P. Hallast, M. Grigorova, M. Laan, Genomics and genetics of gonadotropin beta-subunit genes: unique FSHB and duplicated LHB/CGB loci. Mol. Cell. Endocrinol. 329(1), 4–16 (2010)
A.W. Norman, H.L. Henry, Hormones (Academic Press, San Diego, 2014)
L. O’Donnell, S.J. Meachem, P.G. Stanton, R.I. McLachlan, in Chapter 21: Endocrine Regulation of Spermatogenesis. ed. by J.D. Neill, T.M. Plant, D.W. Pfaff, J.R.G. Challis, D.M. de Kretser, J.S. Richards, P.M. Wassarman. Knobil and Neill’s Physiology of Reproduction, vol 1 (Academic Press, St. Louis, 2006), pp. 1017–1069
K.-H. Jeong, U.B. Kaiser, in Chapter 31: Gonadotropin-Releasing Hormone Regulation of Gonadotropin Biosynthesis and Secretion. ed. by J.D. Neill, T.M. Plant, D.W. Pfaff, J.R.G. Challis, D.M. de Kretser, J.S. Richards, P.M. Wassarman (Academic Press, St. Louis, 2006), pp. 1635–1701
A.E. O’Connor, D.M. De Kretser, Inhibins in normal male physiology. Semin. Reprod. Med. 22(3), 177–185 (2004). https://doi.org/10.1055/s-2004-831893
J.G. Pierce, T.F. Parsons, Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50(1), 465–495 (1981)
F.J. Hayes, S. DeCruz, S.B. Seminara, P.A. Boepple, W.F. Crowley Jr., Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J. Clin. Endocrinol. Metab. 86(1), 53–58 (2001). https://doi.org/10.1210/jcem.86.1.7101
M.R. Laurent, G.L. Hammond, M. Blokland, F. Jardi, L. Antonio, V. Dubois, R. Khalil, S.S. Sterk, E. Gielen, B. Decallonne, G. Carmeliet, J.M. Kaufman, T. Fiers, I.T. Huhtaniemi, D. Vanderschueren, F. Claessens, Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci. Rep. 6, 35539 (2016). https://doi.org/10.1038/srep35539
Y. Li, X. Li, H. Fan, X. Li, Y. Zhong, J. Cao, D. Yu, M. Zhang, J.G. Wen, L. Geng, Z. Suo, Age-dependent sex hormone-binding globulin expression in male rat. Ultrastruct. Pathol. 39(2), 121–130 (2015). https://doi.org/10.3109/01913123.2015.1009222
P.J. Davis, F. Goglia, J.L. Leonard, Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 12(2), 111–121 (2016). https://doi.org/10.1038/nrendo.2015.205
P. Bargi-Souza, R.M. Romano, F. Goulart-Silva, E.L. Brunetto, M.T. Nunes, T(3) rapidly regulates several steps of alpha subunit glycoprotein (CGA) synthesis and secretion in the pituitary of male rats: potential repercussions on TSH, FSH and LH secretion. Mol. Cell. Endocrinol. 409, 73–81 (2015). https://doi.org/10.1016/j.mce.2015.04.002
W. Rosner, D.P. Aden, M.S. Khan, Hormonal influences on the secretion of steroid-binding proteins by a human hepatoma-derived cell line. J. Clin. Endocrinol. Metab. 59(4), 806–808 (1984). https://doi.org/10.1210/jcem-59-4-806
Y. Cai, M.M. Manio, G.P. Leung, A. Xu, E.H. Tang, P.M. Vanhoutte, Thyroid hormone affects both endothelial and vascular smooth muscle cells in rat arteries. Eur. J. Pharmacol. 747, 18–28 (2015). https://doi.org/10.1016/j.ejphar.2014.11.036
A.L. de Castro, A.V. Tavares, R.O. Fernandes, C. Campos, A. Conzatti, R. Siqueira, T.R. Fernandes, P.C. Schenkel, C.L. Sartorio, S. Llesuy, A. Bello-Klein, A.S. da Rosa Araujo, T3 and T4 decrease ROS levels and increase endothelial nitric oxide synthase expression in the myocardium of infarcted rats. Mol. Cell. Biochem 408(1–2), 235–243 (2015). https://doi.org/10.1007/s11010-015-2501-4
D.J. Grieve, S. Fletcher, A.A. Pitsillides, K.M. Botham, J. Elliott, Effects of oral propylthiouracil treatment on nitric oxide production in rat aorta. Br. J. Pharmacol. 127(1), 1–8 (1999). https://doi.org/10.1038/sj.bjp.0702501
L. Tian, L. Zhang, J. Liu, T. Guo, C. Gao, J. Ni, Effects of TSH on the function of human umbilical vein endothelial cells. J. Mol. Endocrinol. 52(2), 215–222 (2014). https://doi.org/10.1530/jme-13-0119
E. Bussemaker, R. Popp, B. Fisslthaler, C.M. Larson, I. Fleming, R. Busse, R.P. Brandes, Hyperthyroidism enhances endothelium-dependent relaxation in the rat renal artery. Cardiovasc. Res. 59(1), 181–8 (2003)
U. Förstermann, W.C. Sessa, Nitric oxide synthases: regulation and function. Eur. Heart J. 33(7), 829–837 (2012). https://doi.org/10.1093/eurheartj/ehr304
R. Ramachandran, K.B. Ploug, A. Hay-Schmidt, J. Olesen, I. Jansen-Olesen, S. Gupta, Nitric oxide synthase (NOS) in the trigeminal vascular system and other brain structures related to pain in rats. Neurosci. Lett. 484(3), 192–196 (2010). https://doi.org/10.1016/j.neulet.2010.08.050
M.C. Franco, V.G. Antico Arciuch, J.G. Peralta, S. Galli, D. Levisman, L.M. Lopez, L. Romorini, J.J. Poderoso, M.C. Carreras, Hypothyroid phenotype is contributed by mitochondrial complex I inactivation due to translocated neuronal nitric-oxide synthase. J. Biol. Chem. 281(8), 4779–4786 (2006). https://doi.org/10.1074/jbc.M512080200
T. Song, N. Hatano, T. Kambe, Y. Miyamoto, H. Ihara, H. Yamamoto, K. Sugimoto, K. Kume, F. Yamaguchi, M. Tokuda, Y. Watanabe, Nitric oxide-mediated modulation of calcium/calmodulin-dependent protein kinase II. Biochem. J. 412(2), 223–231 (2008). https://doi.org/10.1042/bj20071195
Acknowledgements
The authors thank Leonice Lourenço Poyares for the excellent technical assistance.
Funding
This work was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP, (2008/50977-2, 2009/17822-8, 2013/05629-4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (305936/2013-1), Brazil. P.B.S., R.M.R., and E.L.B. are the recipients of a FAPESP fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing of interests.
Ethical approval
All procedures are in accordance to the Brazilian College of Animal Experimentation and approved by the Institute of Biomedical Sciences, University of Sao Paulo—Ethical Committee for Animal Research (protocol 029/55/02).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Romano, R.M., Bargi-Souza, P., Brunetto, E.L. et al. Triiodothyronine differentially modulates the LH and FSH synthesis and secretion in male rats. Endocrine 59, 191–202 (2018). https://doi.org/10.1007/s12020-017-1487-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1487-y