Abstract
Introduction
Gastric neuro-endocrine tumours are rare. European guidelines for the management of neuro-endocrine tumours have been published in 2012. The aim of our survey was to study the management of gastric neuro-endocrine tumours registered in the national cohort. A prospective national cohort registers the Neuro-endocrine tumours in France since January 2003 (GTE network). We reviewed all the individual medical reports of gastric neuro-endocrine tumours in order to collect data on treatment.
Results
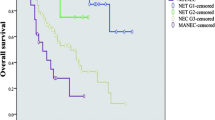
One hundred and ninety seven gastric neuro-endocrine tumours diagnosed between 1964 and 2013 in 20 centres were registered. For 181 cases data were considered complete for our survey. Eighty four tumours were type 1 (46.4%); five types 2 (2.8%); 52 types 3 (28.7%) and 40 types 4 (22.1%). Types 1 and 2 were first endoscopically managed in 93 and 60% of cases, respectively, whereas surgery was first done in 45 and 42%, respectively, of types 3 and 4. Systemic treatment, chemotherapy and/or somatostatin analogue, was first administered exclusively for types 3 and 4. Near 3% of types 1 and 40% of types 2 received at a time somatostatin analogue treatment. Five-year survival rates were 98.3, 100, 63.2 and 31.8% for types 1, 2, 3 and 4, respectively.
Conclusion
The great majority of gastric neuro-endocrine tumours registered in this national cohort are treated in accordance with the current guidelines. The survival rates we reported must be interpreted with caution, because this cohort registered preferentially selected patients eligible for treatment. The registration of all the gastric neuro-endocrine tumours, in particular type 1 considered as benign and type 4 not eligible for specific anti-cancer treatment must be encouraged.


Similar content being viewed by others
References
M. Fraenkel, M.K. Kim, A. Faggiano, G.D. Valk, Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 26, 691–703 (2012)
C. Lepage, A.M. Bouvier, J.M. Phelip, C. Hatem, C. Vernet, J. Faivre, Incidence and management of malignant digestive endocrine tumours in a well-defined French population. Gut 53, 549–553 (2004)
I.M. Modlin, K.D. Lye, M. Kidd, A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am. J. Gastroenterol. 99, 23–32 (2004)
C.S. Landry, G. Brock, C.R. Scoggins, K.M. McMasters, R.C. Martin 2nd, A proposed staging system for gastric carcinoid tumors based on an analysis of 1,543 patients. Ann. Surg. Oncol. 16, 51–60 (2009)
M.B. Niederle, M. Hackl, K. Kaserer, B. Niederle, Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European neuroendocrine tumour society classification: an analysis based on prospectively collected parameters. Endocr. Relat. Cancer 17, 909–918 (2010)
L. Vannella, A. Sbrozzi-Vanni, E. Lahner et al., Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment. Pharmacol. Ther. 33, 1361–1369 (2011)
F. Gibril, M. Schumann, A. Pace et al., Multiple endocrine neoplasia type 1 and Zollinger- Ellison syndrome: a prospective study of 107 cases and comparison with 1,009 cases from the literature. Medicine 83, 43–83 (2004)
K. Borch, B. Ahren, H. Ahlman et al., Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann. Surg. 242, 64–73 (2005)
G. Rindi, C. Azzoni, S. La Rosa et al., ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology 116, 532–542 (1999)
H. Scherübl, G. Cadiot, R.T. Jensen, T. Rösch, U. Stölzel, G. Klöppel, Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy 42, 664–671 (2010)
S. Rappel, A. Altendorf-Hofmann, M. Stolte, Prognosis of gastric carcinoid tumours. Digestion 56, 455–462 (1995)
Y. Sato, H. Imamura, Y. Kaizaki et al., Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig. Endosc. 26, 377–384 (2014)
D. Thomas, A.V. Tsolakis, S. Grozinsky-Glasberg, M. Fraenkel, K. Alexandraki, S. Sougioultzis, D.J. Gross, G. Kaltsas, Long-term follow-up of a large series of patients with type 1 gastric carcinoid tumors: data from a multicenter study. Eur. J. Endocrinol. 168, 185–193 (2013)
E. Merola, A. Sbrozzi-Vanni, F. Panzuto et al., Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 95, 207–213 (2012)
W.C. Chen, R.R. Warner, S.C. Ward et al., Management and disease outcome of type I gastric neuroendocrine tumors: the Mount Sinai experience. Dig. Dis. Sci. 60, 996–1003 (2015)
Y. Sato, S. Hashimoto, K. Mizuno, M. Takeuchi, S. Terai, Management of gastric and duodenal neuroendocrine tumors. World J. Gastroenterol. 22, 6817–6828 (2016)
G. Delle Fave, D.J. Kwekkeboom, E. Van Cutsem, G. Rindi, B. Kos-Kudla, U. Knigge, H. Sasano, P. Tomassetti, R. Salazar, P. Ruszniewski, ENETS consensus guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology 95, 74–87 (2012)
E. Solcia, G. Kloppel, L.H. Sobin, Histological typing of endocrine tumours. In international histological classification of tumours, 2nd edn. (Springer, Berlin Heidelberg, 2000) pp. 61–68
L.H. Sobin, M. Gospodarowicz, C. Wittekind (eds.), UICC (International Union Against Cancer). TNM classification of malignant tumours. 7th edn. (Wiley-Blackwell, New York, 2009)
C. Lepage, B. Rachet, M.P. Coleman, Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology 132, 899–904 (2007)
C. Lepage, L. Ciccolallo, R. De Angelis, A.M. Bouvier, J. Faivre, G. Gatta, The EUROCARE working group, European disparities in malignant digestive endocrine tumours survival. Int. J. Cancer 126, 2928–2934 (2010)
M.H. Kulke, L.B. Anthony, D.L. Bushnell, W.W. de Herder, S.J. Goldsmith, D.S. Klimstra, S.J. Marx, J.L. Pasieka, R.F. Pommier, J.C. Yao, R.T. Jensen, North American neuroendocrine tumor society (NANETS), NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 39, 735–752 (2010)
R.A. Gladdy, V.E. Strong, D. Coit, P.J. Allen, H. Gerdes, J. Shia, D.S. Klimstra, F.B. Murray, L.H. Tang, Defining surgical indications for type I gastric carcinoid tumor. Ann. Surg. Oncol. 16, 3154–3160 (2009)
D. Ravizza, G. Fiori, C. Trovato, N. Fazio, G. Bonomo, F. Luca, L. Boder, G. Pelosi, D. Tamayo, C. Crosta, Long-term endoscopic and clinical follow-up of untreated type 1 gastric neuroendocrine tumours. Dig. Liver. Dis. 39, 537–543 (2007)
S. Manfredi, M. Pagenault, A.S. de Lajarte-Thirouard, J.F. Bretagne, Type 1 and 2 gastric carcinoid tumors: long-term follow-up of the efficacy of treatment with a slow-release somatostatin analogue. Eur. J. Gastroenterol. Hepatol. 19, 1021–1025 (2007)
S. Grozinsky-Glasberg, G. Kaltsas, C. Gur, E. Gal, D. Thomas, S. Fichman, K. Alexandraki, D. Barak, B. Glaser, I. Shimon, D.J. Gross, Long-acting somatostatin analogues are an effective treatment for type 1 gastric carcinoid tumours. Eur. J. Endocrinol. 159, 475–482 (2008)
D. Campana, F. Nori, R. Pezzilli, L. Piscitelli, D. Santini, E. Brocchi, R. Corinaldesi, P. Tomassetti, Gastric endocrine tumors type I: treatment with long-acting somatostatin analogs. Endocr. Relat. Cancer 15, 337–342 (2008)
M.S. Khuroo, M.S. Khuroo, N.S. Khuroo, Treatment of type I gastric neuroendocrine tumors with somatostatin analogs. J. Gastroenterol. Hepatol. 25, 548–554 (2010)
P. Tomassetti, M. Migliori, G.C. Caletti, P. Fusaroli, R. Corinaldesi, L. Gullo, Treatment of type II gastric carcinoid tumors with somatostatin analogues. N. Engl. J. Med. 343, 551–554 (2000)
D. Campana, D. Ravizza, P. Ferolla, A. Faggiano, F. Grimaldi, M. Albertelli, D. Berretti, D. Castellani, G. Cacciari, N. Fazio, A. Colao, D. Ferone, P. Tomassetti, Clinical management of patients with gastric neuroendocrine neoplasms associated with chronic atrophic gastritis: a retrospective, multicentre study. Endocrine 51, 131–139 (2016)
S. Massironi, V. Sciola, M. Pia Spampatti, M. Peracchi, D. Conte, Gastric carcinoids: between underestimation and overtreatment. World J. Gastroenterol. 15, 2177–2183 (2009)
A. Rinke, H.H. Müller, C. Schade-Brittinger, K.J. Klose, P. Barth, M. Wied, C. Mayer, B. Aminossadati, U.F. Pape, M. Bläker, J. Harder, C. Arnold, T. Gress, R. Arnold, PROMID Study Group, Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J. Clin. Oncol. 27, 4656–4663 (2009)
M.E. Caplin, M. Pavel, J.B. Ćwikła, A.T. Phan, M. Raderer, E. Sedláčková, G. Cadiot, E.M. Wolin, J. Capdevila, L. Wall, G. Rindi, A. Langley, S. Martinez, J. Blumberg, P. Ruszniewski, CLARINET Investigators, Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 371, 224–233 (2014)
D. O’Toole, G. Delle Fave, R.T. Tensen, Gastric and duodenal neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 26, 719–735 (2012)
Acknowledgements
We are thankful to all the members of the two academics networks involved in this cohort: the GTE (group for the study of endocrine tumours) and RENATEN (national network for the treatment of endocrine tumours). All the patients and their family who gave consents for this study.
Funding
The study was supported by a fund from Ipsen Pharma SAS.
Author contributions
S.M.: drafted and designed the study, recruited patients, and wrote the paper. T.W., G.C.: recruited patients, revised the manuscript; E.B., R.C., P.R., T.L., A.P.L., B.G., V.R., G.R., O.Z.C., C.L.B., C.L.: recruited patients. J.M.: did the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the scientific committee of the national group for NETs (GTE) and the ethic committee of the university hospital of Rennes, France.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Manfredi, S., Walter, T., Baudin, E. et al. Management of gastric neuro-endocrine tumours in a large French national cohort (GTE). Endocrine 57, 504–511 (2017). https://doi.org/10.1007/s12020-017-1355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1355-9