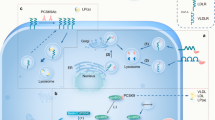
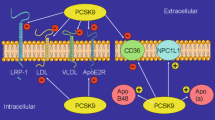
Abstract
Low-density lipoprotein (LDL) cholesterol plays a pivotal role in the pathogenesis of atherosclerotic cardiovascular disease (CVD). The discovery that proprotein convertase subtilisin/kexin type 9 (PCSK9) represents a key regulator pathway for hepatic LDL receptor (LDLR) degradation sheds light on new uncovered issues regarding LDL-C homeostasis. Indeed, as confirmed by phase II and III clinical trials with monoclonal antibodies, targeting PCSK9 represents the newest and most promising pharmacological tool for the treatment of hypercholesterolemia and related CVD. However, clinical, genetic, and experimental evidence indicates that PCSK9 may be either a cause or an effect in the context of metabolic syndrome (MetS), a condition comprising a cluster of risk factors including insulin resistance, obesity, hypertension, and atherogenic dyslipidemia. The latter is characterized by a triad of hypertriglyceridemia, low plasma concentrations of high-density lipoproteins, and qualitative changes in LDLs. PCSK9 levels seem to correlate with many of these lipid parameters as well as with the insulin sensitivity indices, although the molecular mechanisms behind this association are still unknown or not completely elucidated. Nevertheless, this area of research represents an important starting point for a better understanding of the physiological role of PCSK9, also considering the recent approval of new therapies involving anti-PCSK9. Thus, in the present review, we will discuss the current knowledge on the role of PCSK9 in the context of MetS, alteration of lipids, glucose homeostasis, and inflammation.


Similar content being viewed by others
References
S.M. Grundy, Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 28(4), 629–636 (2008). doi:10.1161/ATVBAHA.107.151092
K.G. Alberti, R.H. Eckel, S.M. Grundy, P.Z. Zimmet, J.I. Cleeman, K.A. Donato, J.C. Fruchart, W.P. James, C.M. Loria, S.C. Smith Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity, Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16), 1640–1645 (2009). doi:10.1161/CIRCULATIONAHA.109.192644
Cholesterol Treatment Trialists, P.M. Kearney, L. Blackwell, R. Collins, A. Keech, J. Simes, R. Peto, J. Armitage, C. Baigent, Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 371(9607), 117–125 (2008). doi:10.1016/S0140-6736(08)60104-X
A.L. Catapano, Z. Reiner, G. De Backer, I. Graham, M.R. Taskinen, O. Wiklund, S. Agewall, E. Alegria, M. Chapman, P. Durrington, S. Erdine, J. Halcox, R. Hobbs, J. Kjekshus, P.P. Filardi, G. Riccardi, R.F. Storey, D. Wood, European Society of Cardiology, European Atherosclerosis Society, ESC/EAS Guidelines for the management of dyslipidaemias. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 217(1), 3–46 (2011)
R. Scott, R. O’Brien, G. Fulcher, C. Pardy, M. D’Emden, D. Tse, M.R. Taskinen, C. Ehnholm, A. Keech, I. Fenofibrate, Event lowering in diabetes study. I: Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 32(3), 493–498 (2009). doi:10.2337/dc08-1543
M.E. Haas, A.D. Attie, S.B. Biddinger, The regulation of ApoB metabolism by insulin. Trends Endocrinol. Metab. 24(8), 391–397 (2013). doi:10.1016/j.tem.2013.04.001
M.P. van der Aa, S. Fazeli Farsani, L.A. Kromwijk, A. de Boer, C.A. Knibbe, M.M. van der Vorst, How to screen obese children at risk for type 2 diabetes mellitus? Clin. Pediatr. 53(4), 337–342 (2014). doi:10.1177/0009922813509480
H.N. Ginsberg, Insulin resistance and cardiovascular disease. J. Clin. Invest. 106(4), 453–458 (2000). doi:10.1172/JCI10762
H.N. Ginsberg, L.S. Huang, The insulin resistance syndrome: impact on lipoprotein metabolism and atherothrombosis. J. Cardiovasc. Risk 7(5), 325–331 (2000)
I. Tabas, K.J. Williams, J. Boren, Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116(16), 1832–1844 (2007). doi:10.1161/CIRCULATIONAHA.106.676890
J.D. Sparks, C.E. Sparks, K. Adeli, Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 32(9), 2104–2112 (2012). doi:10.1161/ATVBAHA.111.241463
F.K. Welty, A. Alfaddagh, T.K. Elajami, Targeting inflammation in metabolic syndrome. Transl. Res. 167(1), 257–280 (2016). doi:10.1016/j.trsl.2015.06.017
M.M. van Greevenbroek, C.G. Schalkwijk, C.D. Stehouwer, Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth. J. Med. 71(4), 174–187 (2013)
N. Kloting, M. Bluher, Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 15(4), 277–287 (2014). doi:10.1007/s11154-014-9301-0
P. Libby, P.M. Ridker, A. Maseri, Inflammation and atherosclerosis. Circulation 105(9), 1135–1143 (2002)
E. Chernogubova, R. Strawbridge, H. Mahdessian, A. Malarstig, S. Krapivner, B. Gigante, M.L. Hellenius, U. de Faire, A. Franco-Cereceda, A.C. Syvanen, J.S. Troutt, R.J. Konrad, P. Eriksson, A. Hamsten, F.M. van’t Hooft, Common and low-frequency genetic variants in the PCSK9 locus influence circulating PCSK9 levels. Arterioscler. Thromb. Vasc. Biol. 32(6), 1526–1534 (2012). doi:10.1161/ATVBAHA.111.240549
M. Ghosh, C. Galman, M. Rudling, B. Angelin, Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J. Lipid Res. 56(2), 463–469 (2015). doi:10.1194/jlr.M055780
Q. Feng, W.Q. Wei, C.P. Chung, R.T. Levinson, L. Bastarache, J.C. Denny, C.M. Stein, The effect of genetic variation in PCSK9 on the LDL-cholesterol response to statin therapy. Pharmacogenomics J. (2016). doi:10.1038/tpj.2016.3
N.G. Seidah, S. Benjannet, L. Wickham, J. Marcinkiewicz, S.B. Jasmin, S. Stifani, A. Basak, A. Prat, M. Chretien, The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. U.S.A. 100(3), 928–933 (2003)
G. Lambert, B. Sjouke, B. Choque, J.J. Kastelein, G.K. Hovingh, The PCSK9 decade. J. Lipid Res. 53(12), 2515–2524 (2012). doi:10.1194/jlr.R026658
S. Poirier, G. Mayer, S. Benjannet, E. Bergeron, J. Marcinkiewicz, N. Nassoury, H. Mayer, J. Nimpf, A. Prat, N.G. Seidah, The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 283(4), 2363–2372 (2008)
N. Ferri, A. Corsini, C. Macchi, P. Magni, M. Ruscica, Proprotein convertase subtilisin kexin type 9 and high-density lipoprotein metabolism: experimental animal models and clinical evidence. Transl. Res. (2015). doi:10.1016/j.trsl.2015.10.004
S. Henrich, I. Lindberg, W. Bode, M.E. Than, Proprotein convertase models based on the crystal structures of furin and kexin: explanation of their specificity. J. Mol. Biol. 345(2), 211–227 (2005)
D. Cunningham, D.E. Danley, K.F. Geoghegan, M.C. Griffor, J.L. Hawkins, T.A. Subashi, A.H. Varghese, M.J. Ammirati, J.S. Culp, L.R. Hoth, M.N. Mansour, K.M. McGrath, A.P. Seddon, S. Shenolikar, K.J. Stutzman-Engwall, L.C. Warren, D. Xia, X. Qiu, Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 14(5), 413–419 (2007). doi:10.1038/nsmb1235
N.G. Seidah, A. Prat, Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 38, 79–94 (2002)
Z. Awan, A. Baass, J. Genest, Proprotein convertase subtilisin/kexin type 9 (PCSK9): lessons learned from patients with hypercholesterolemia. Clin. Chem. 60(11), 1380–1389 (2014). doi:10.1373/clinchem.2014.225946
A.S. Peterson, L.G. Fong, S.G. Young, PCSK9 function and physiology. J. Lipid Res. 49(7), 1595–1599 (2008)
J.L. Goldstein, M.S. Brown, The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29(4), 431–438 (2009). doi:10.1161/ATVBAHA.108.179564
Y.W. Qian, R.J. Schmidt, Y. Zhang, S. Chu, A. Lin, H. Wang, X. Wang, T.P. Beyer, W.R. Bensch, W. Li, M.E. Ehsani, D. Lu, R.J. Konrad, P.I. Eacho, D.E. Moller, S.K. Karathanasis, G. Cao, Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J. Lipid Res. 48(7), 1488–1498 (2007)
H.J. Kwon, T.A. Lagace, M.C. McNutt, J.D. Horton, J. Deisenhofer, Molecular basis for LDL receptor recognition by PCSK9. Proc. Natl. Acad. Sci. U.S.A. 105(6), 1820–1825 (2008)
M. Abifadel, S. Elbitar, P. El Khoury, Y. Ghaleb, M. Chemaly, M.L. Moussalli, J.P. Rabes, M. Varret, C. Boileau, Living the PCSK9 adventure: from the identification of a new gene in familial hypercholesterolemia towards a potential new class of anticholesterol drugs. Curr. Atheroscler. Rep. 16(9), 439 (2014). doi:10.1007/s11883-014-0439-8
T.B. Strom, K. Tveten, T.P. Leren, PCSK9 acts as a chaperone for the LDL receptor in the endoplasmic reticulum. Biochem. J. 457(1), 99–105 (2014). doi:10.1042/BJ20130930
S. Poirier, G. Mayer, V. Poupon, P.S. McPherson, R. Desjardins, K. Ly, M.C. Asselin, R. Day, F.J. Duclos, M. Witmer, R. Parker, A. Prat, N.G. Seidah, Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J. Biol. Chem. 284(42), 28856–28864 (2009)
H.E. Careskey, R.A. Davis, W.E. Alborn, J.S. Troutt, G. Cao, R.J. Konrad, Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 49(2), 394–398 (2008). doi:10.1194/jlr.M700437-JLR200
H.J. Jeong, H.S. Lee, K.S. Kim, Y.K. Kim, D. Yoon, S.W. Park, Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J. Lipid Res. 49(2), 399–409 (2008). doi:10.1194/jlr.M700443-JLR200
H. Li, B. Dong, S.W. Park, H.S. Lee, W. Chen, J. Liu, Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 284(42), 28885–28895 (2009). doi:10.1074/jbc.M109.052407
M.S. Brown, J.L. Goldstein, The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89(3), 331–340 (1997)
D. Eberle, B. Hegarty, P. Bossard, P. Ferre, F. Foufelle, SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86(11), 839–848 (2004). doi:10.1016/j.biochi.2004.09.018
J.D. Horton, N.A. Shah, J.A. Warrington, N.N. Anderson, S.W. Park, M.S. Brown, J.L. Goldstein, Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. U.S.A. 100(21), 12027–12032 (2003)
C. Pramfalk, Z.Y. Jiang, Q. Cai, H. Hu, S.D. Zhang, T.Q. Han, M. Eriksson, P. Parini, HNF1alpha and SREBP2 are important regulators of NPC1L1 in human liver. J. Lipid Res. 51(6), 1354–1362 (2010). doi:10.1194/jlr.M900274
V.R. Shende, M. Wu, A.B. Singh, B. Dong, C.F. Kan, J. Liu, Reduction of circulating PCSK9 and LDL-C levels by liver-specific knockdown of HNF1alpha in normolipidemic mice. J. Lipid Res. 56(4), 801–809 (2015). doi:10.1194/jlr.M052969
P. Costet, B. Cariou, G. Lambert, F. Lalanne, B. Lardeux, A.L. Jarnoux, A. Grefhorst, B. Staels, M. Krempf, Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J. Biol. Chem. 281(10), 6211–6218 (2006)
L. Persson, G. Cao, L. Stahle, B.G. Sjoberg, J.S. Troutt, R.J. Konrad, C. Galman, H. Wallen, M. Eriksson, I. Hafstrom, S. Lind, M. Dahlin, P. Amark, B. Angelin, M. Rudling, Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler. Thromb. Vasc. Biol. 30(12), 2666–2672 (2010). doi:10.1161/ATVBAHA.110.214130
J.D. Browning, J.D. Horton, Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J. Lipid Res. 51(11), 3359–3363 (2010). doi:10.1194/jlr.P009860
C. Richard, P. Couture, S. Desroches, S. Benjannet, N.G. Seidah, A.H. Lichtenstein, B. Lamarche, Effect of the Mediterranean diet with and without weight loss on surrogate markers of cholesterol homeostasis in men with the metabolic syndrome. Br. J. Nutr. 107(5), 705–711 (2012). doi:10.1017/S0007114511003436
C. Rodriguez-Perez, V.R. Ramprasath, S. Pu, A. Sabra, R. Quirantes-Pine, A. Segura-Carretero, P.J. Jones, Docosahexaenoic acid attenuates cardiovascular risk factors via a decline in proprotein convertase subtilisin/kexin type 9 (PCSK9) plasma levels. Lipids 51(1), 75–83 (2016). doi:10.1007/s11745-015-4099-4
H. Bjermo, D. Iggman, J. Kullberg, I. Dahlman, L. Johansson, L. Persson, J. Berglund, K. Pulkki, S. Basu, M. Uusitupa, M. Rudling, P. Arner, T. Cederholm, H. Ahlstrom, U. Riserus, Effects of n − 6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am. J. Clin. Nutr. 95(5), 1003–1012 (2012). doi:10.3945/ajcn.111.030114
P. Simonen, U.H. Stenman, H. Gylling, Serum proprotein convertase subtilisin/kexin type 9 concentration is not increased by plant stanol ester consumption in normo- to moderately hypercholesterolaemic non-obese subjects. The BLOOD FLOW intervention study. Clin. Sci. 129(5), 439–446 (2015). doi:10.1042/CS20150193
M. Rudling, B. Angelin, Stimulation of rat hepatic low density lipoprotein receptors by glucagon. Evidence of a novel regulatory mechanism in vivo. J. Clin. Invest. 91(6), 2796–2805 (1993). doi:10.1172/JCI116522
L. Persson, C. Galman, B. Angelin, M. Rudling, Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology 150(3), 1140–1146 (2009). doi:10.1210/en.2008-1281
J. Miao, P.V. Manthena, M.E. Haas, A.V. Ling, D.J. Shin, M.J. Graham, R.M. Crooke, J. Liu, S.B. Biddinger, Role of insulin in the regulation of proprotein convertase subtilisin/kexin type 9. Arterioscler. Thromb. Vasc. Biol. 35(7), 1589–1596 (2015). doi:10.1161/ATVBAHA.115.305688
H. Khodabandehloo, S. Gorgani-Firuzjaee, G. Panahi, R. Meshkani, Molecular and cellular mechanisms linking inflammation to insulin resistance and beta-cell dysfunction. Transl. Res. 167(1), 228–256 (2016). doi:10.1016/j.trsl.2015.08.011
L. Patel, A.C. Buckels, I.J. Kinghorn, P.R. Murdock, J.D. Holbrook, C. Plumpton, C.H. Macphee, S.A. Smith, Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 300(2), 472–476 (2003)
S. Rashid, J.J. Kastelein, PCSK9 and resistin at the crossroads of the atherogenic dyslipidemia. Expert Rev. Cardiovasc. Ther. 11(11), 1567–1577 (2013). doi:10.1586/14779072.2013.839204
P. Codoner-Franch, E. Alonso-Iglesias, Resistin: insulin resistance to malignancy. Clin. Chim. Acta 438, 46–54 (2015). doi:10.1016/j.cca.2014.07.043
E.N. Hampton, M.W. Knuth, J. Li, J.L. Harris, S.A. Lesley, G. Spraggon, The self-inhibited structure of full-length PCSK9 at 1.9 A reveals structural homology with resistin within the C-terminal domain. Proc. Natl. Acad. Sci. U.S.A. 104(37), 14604–14609 (2007)
M. Melone, L. Wilsie, O. Palyha, A. Strack, S. Rashid, Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. J. Am. Coll. Cardiol. 59(19), 1697–1705 (2012)
M. Ruscica, C. Ricci, C. Macchi, P. Magni, R. Cristofani, J. Liu, A. Corsini, N. Ferri, Suppressor of cytokine signaling-3 (SOCS-3) induces proprotein convertase subtilisin kexin type 9 (PCSK9) expression in hepatic HepG2 cell line. J. Biol. Chem. (2015). doi:10.1074/jbc.M115.664706
S.G. Lakoski, T.A. Lagace, J.C. Cohen, J.D. Horton, H.H. Hobbs, Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 94(7), 2537–2543 (2009)
L. Persson, P. Henriksson, E. Westerlund, O. Hovatta, B. Angelin, M. Rudling, Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arterioscler. Thromb. Vasc. Biol. 32(3), 810–814 (2012). doi:10.1161/ATVBAHA.111.242461
G.F. Lewis, G. Steiner, Hypertriglyceridemia and its metabolic consequences as a risk factor for atherosclerotic cardiovascular disease in non-insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 12(1), 37–56 (1996). doi:10.1002/(SICI)1099-0895(199603)12:1<37:AID-DMR154>3.0.CO;2-Q
K. Adeli, C. Taghibiglou, S.C. Van Iderstine, G.F. Lewis, Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc. Med. 11(5), 170–176 (2001)
F. Shojaee-Moradie, Y. Ma, S. Lou, R. Hovorka, A.M. Umpleby, Prandial hypertriglyceridemia in metabolic syndrome is due to an overproduction of both chylomicron and VLDL triacylglycerol. Diabetes 62(12), 4063–4069 (2013). doi:10.2337/db13-0935
W.E. Alborn, G. Cao, H.E. Careskey, Y.W. Qian, D.R. Subramaniam, J. Davies, E.M. Conner, R.J. Konrad, Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin. Chem. 53(10), 1814–1819 (2007)
G. Dubuc, M. Tremblay, G. Pare, H. Jacques, J. Hamelin, S. Benjannet, L. Boulet, J. Genest, L. Bernier, N.G. Seidah, J. Davignon, A new method for measurement of total plasma PCSK9: clinical applications. J. Lipid Res. 51(1), 140–149 (2010). doi:10.1194/jlr.M900273-JLR200
A. Baass, G. Dubuc, M. Tremblay, E.E. Delvin, J. O’Loughlin, E. Levy, J. Davignon, M. Lambert, Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin. Chem. 55(9), 1637–1645 (2009). doi:10.1373/clinchem.2009.126987
S.H. Yang, S. Li, Y. Zhang, R.X. Xu, Y.L. Guo, C.G. Zhu, N.Q. Wu, C.J. Cui, J. Sun, J.J. Li, Positive correlation of plasma PCSK9 levels with HbA in patients with type 2 diabetes. Diabetes/Metab. Res. Rev. (2015). doi:10.1002/dmrr.2712
B. Cariou, C. Langhi, M. Le Bras, M. Bortolotti, K.A. Le, F. Theytaz, C. Le May, B. Guyomarc’h-Delasalle, Y. Zair, R. Kreis, C. Boesch, M. Krempf, L. Tappy, P. Costet, Plasma PCSK9 concentrations during an oral fat load and after short term high-fat, high-fat high-protein and high-fructose diets. Nutr. Metab. 10(1), 4 (2013). doi:10.1186/1743-7075-10-4
L. Tappy, K.A. Le, C. Tran, N. Paquot, Fructose and metabolic diseases: new findings, new questions. Nutrition 26(11–12), 1044–1049 (2010). doi:10.1016/j.nut.2010.02.014
P.J. Kappelle, G. Lambert, R.P. Dullaart, Plasma proprotein convertase subtilisin-kexin type 9 does not change during 24 h insulin infusion in healthy subjects and type 2 diabetic patients. Atherosclerosis 214(2), 432–435 (2011). doi:10.1016/j.atherosclerosis.2010.10.028
Z. Awan, G. Dubuc, M. Faraj, R. Dufour, N.G. Seidah, J. Davignon, R. Rabasa-Lhoret, A. Baass, The effect of insulin on circulating PCSK9 in postmenopausal obese women. Clin. Biochem. 47(12), 1033–1039 (2014). doi:10.1016/j.clinbiochem.2014.03.022
M.C. Brouwers, J.S. Troutt, M.M. van Greevenbroek, I. Ferreira, E.J. Feskens, C.J. van der Kallen, N.C. Schaper, C.G. Schalkwijk, R.J. Konrad, C.D. Stehouwer, Plasma proprotein convertase subtilisin kexin type 9 is not altered in subjects with impaired glucose metabolism and type 2 diabetes mellitus, but its relationship with non-HDL cholesterol and apolipoprotein B may be modified by type 2 diabetes mellitus: the CODAM study. Atherosclerosis 217(1), 263–267 (2011). doi:10.1016/j.atherosclerosis.2011.03.023
B. Verges, L. Duvillard, M.C. Brindisi, E. Gautier, M. Krempf, P. Costet, B. Cariou, Lack of association between plasma PCSK9 and LDL-apoB100 catabolism in patients with uncontrolled type 2 diabetes. Atherosclerosis 219(1), 342–348 (2011)
L. Pisciotta, R. Sallo, C. Rabacchi, A. Wunsch, S. Calandra, S. Bertolini, Leucine 10 allelic variant in signal peptide of PCSK9 increases the LDL cholesterol-lowering effect of statins in patients with familial hypercholesterolaemia. Nutr. Metab. Cardiovasc. Dis. 22(10), 831–835 (2012). doi:10.1016/j.numecd.2011.04.003
Y.G. Saavedra, R. Dufour, A. Baass, Familial hypercholesterolemia: PCSK9 InsLEU genetic variant and prediabetes/diabetes risk. J. Clin. Lipidol. 9(6), 786–793 (2015). doi:10.1016/j.jacl.2015.08.005
Z. Awan, E.E. Delvin, E. Levy, J. Genest, J. Davignon, N.G. Seidah, A. Baass, Regional distribution and metabolic effect of PCSK9 insLEU and R46L gene mutations and apoE genotype. Can. J. Cardiol. 29(8), 927–933 (2013). doi:10.1016/j.cjca.2013.03.004
A. Bonnefond, L. Yengo, C. Le May, F. Fumeron, M. Marre, B. Balkau, G. Charpentier, S. Franc, P. Froguel, B. Cariou, DESIR Study Group, The loss-of-function PCSK9 p. R46L genetic variant does not alter glucose homeostasis. Diabetologia 58(9), 2051–2055 (2015). doi:10.1007/s00125-015-3659-8
J.G. Robinson, M. Farnier, M. Krempf, J. Bergeron, G. Luc, M. Averna, E.S. Stroes, G. Langslet, F.J. Raal, M. El Shahawy, M.J. Koren, N.E. Lepor, C. Lorenzato, R. Pordy, U. Chaudhari, J.J. Kastelein, O.L.T. Investigators, Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372(16), 1489–1499 (2015). doi:10.1056/NEJMoa1501031
M.S. Sabatine, R.P. Giugliano, S.D. Wiviott, F.J. Raal, D.J. Blom, J. Robinson, C.M. Ballantyne, R. Somaratne, J. Legg, S.M. Wasserman, R. Scott, M.J. Koren, E.A. Stein, Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators, Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372(16), 1500–1509 (2015). doi:10.1056/NEJMoa1500858
M. Ruscica, C. Macchi, B. Morlotti, C.R. Sirtori, P. Magni, Statin therapy and related risk of new-onset type 2 diabetes mellitus. Eur. J Intern. Med. 25(5), 401–406 (2014). doi:10.1016/j.ejim.2014.03.003
C. Langhi, C. Le May, V. Gmyr, B. Vandewalle, J. Kerr-Conte, M. Krempf, F. Pattou, P. Costet, B. Cariou, PCSK9 is expressed in pancreatic delta-cells and does not alter insulin secretion. Biochem. Biophys. Res. Commun. 390(4), 1288–1293 (2009)
M. Mbikay, F. Sirois, J. Mayne, G.S. Wang, A. Chen, T. Dewpura, A. Prat, N.G. Seidah, M. Chretien, F.W. Scott, PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett. 584(4), 701–706 (2010). doi:10.1016/j.febslet.2009.12.018
B. Cariou, K. Si-Tayeb, C. Le May, Role of PCSK9 beyond liver involvement. Curr. Opin. Lipidol. 26(3), 155–161 (2015). doi:10.1097/MOL.0000000000000180
J. Spranger, A. Kroke, M. Mohlig, K. Hoffmann, M.M. Bergmann, M. Ristow, H. Boeing, A.F. Pfeiffer, Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52(3), 812–817 (2003)
C. Herder, J. Baumert, B. Thorand, W. Koenig, W. de Jager, C. Meisinger, T. Illig, S. Martin, H. Kolb, Chemokines as risk factors for type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984–2002. Diabetologia 49(5), 921–929 (2006). doi:10.1007/s00125-006-0190-y
X. Wang, W. Bao, J. Liu, Y.Y. Ouyang, D. Wang, S. Rong, X. Xiao, Z.L. Shan, Y. Zhang, P. Yao, L.G. Liu, Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36(1), 166–175 (2013). doi:10.2337/dc12-0702
M. Gerber, A. Boettner, B. Seidel, A. Lammert, J. Bar, E. Schuster, J. Thiery, W. Kiess, J. Kratzsch, Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J. Clin. Endocrinol. Metab. 90(8), 4503–4509 (2005). doi:10.1210/jc.2005-0437
A. Corsini, N. Ferri, M. Cortellaro, Are pleiotropic effects of statins real? Vasc. Health Risk Manag. 3(5), 611–613 (2007)
P.M. Ridker, E. Danielson, F.A. Fonseca, J. Genest, A.M. Gotto Jr, J.J. Kastelein, W. Koenig, P. Libby, A.J. Lorenzatti, J.G. Macfadyen, B.G. Nordestgaard, J. Shepherd, J.T. Willerson, R.J. Glynn, Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 373(9670), 1175–1182 (2009)
F.J. Raal, E.A. Stein, R. Dufour, T. Turner, F. Civeira, L. Burgess, G. Langslet, R. Scott, A.G. Olsson, D. Sullivan, G.K. Hovingh, B. Cariou, I. Gouni-Berthold, R. Somaratne, I. Bridges, R. Scott, S.M. Wasserman, D. Gaudet, RUTHERFORD-2 Investigators, PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 385(9965), 331–340 (2015). doi:10.1016/S0140-6736(14)61399-4
J.J. Kastelein, S.E. Nissen, D.J. Rader, G.K. Hovingh, M.D. Wang, T. Shen, K.A. Krueger, Safety and efficacy of LY3015014, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9): a randomized, placebo-controlled Phase 2 study. Eur. Heart J. (2016). doi:10.1093/eurheartj/ehv707
A. Sahebkar, P. Di Giosia, C.A. Stamerra, D. Grassi, C. Pedone, G. Ferretti, T. Bacchetti, C. Ferri, P. Giorgini, Effect of monoclonal antibodies to PCSK9 on high-sensitivity C-reactive protein levels: a meta-analysis of 16 randomized controlled treatment arms. Br. J. Clin. Pharmacol. (2016). doi:10.1111/bcp.12905
K. Ouguerram, M. Chetiveaux, Y. Zair, P. Costet, M. Abifadel, M. Varret, C. Boileau, T. Magot, M. Krempf, Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arterioscler. Thromb. Vasc. Biol. 24(8), 1448–1453 (2004)
J. Twisk, D.L. Gillian-Daniel, A. Tebon, L. Wang, P.H. Barrett, A.D. Attie, The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 105(4), 521–532 (2000). doi:10.1172/JCI8623
A.J. Kwakernaak, G. Lambert, R.P. Dullaart, Plasma proprotein convertase subtilisin-kexin type 9 is predominantly related to intermediate density lipoproteins. Clin. Biochem. 47(7–8), 679–682 (2014). doi:10.1016/j.clinbiochem.2014.03.008
X.M. Sun, E.R. Eden, I. Tosi, C.K. Neuwirth, D. Wile, R.P. Naoumova, A.K. Soutar, Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum. Mol. Genet. 14(9), 1161–1169 (2005)
S.W. Park, Y.A. Moon, J.D. Horton, Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279(48), 50630–50638 (2004)
H. Sun, A. Samarghandi, N. Zhang, Z. Yao, M. Xiong, B.B. Teng, Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler. Thromb. Vasc. Biol. 32(7), 1585–1595 (2012)
S. Rashid, H. Tavori, P.E. Brown, M.F. Linton, J. He, I. Giunzioni, S. Fazio, Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation 130(5), 431–441 (2014). doi:10.1161/CIRCULATIONAHA.113.006720
H. Tavori, I. Giunzioni, I.M. Predazzi, D. Plubell, J. Miles, R.M. DeVay, H. Liang, S. Rashid, M.F. Linton, S. Fazio, Human PCSK9 promotes hepatic lipogenesis and atherosclerosis development via apoE- and LDLR-mediated mechanisms. Cardiovasc. Res. (2016). doi:10.1093/cvr/cvw053
B. Herbert, D. Patel, S.N. Waddington, E.R. Eden, A. McAleenan, X.M. Sun, A.K. Soutar, Increased secretion of lipoproteins in transgenic mice expressing human D374Y PCSK9 under physiological genetic control. Arterioscler. Thromb. Vasc. Biol. 30(7), 1333–1339 (2010)
A. Roubtsova, M.N. Munkonda, Z. Awan, J. Marcinkiewicz, A. Chamberland, C. Lazure, K. Cianflone, N.G. Seidah, A. Prat, Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler. Thromb. Vasc. Biol. 31(4), 785–791 (2011)
D.C. Chan, A.T. Wong, J. Pang, P.H. Barrett, G.F. Watts, Inter-relationships between proprotein convertase subtilisin/kexin type 9, apolipoprotein C-III and plasma apolipoprotein B-48 transport in obese subjects: a stable isotope study in the postprandial state. Clin. Sci. 128(6), 379–385 (2015). doi:10.1042/CS20140559
C. Le May, S. Kourimate, C. Langhi, M. Chetiveaux, A. Jarry, C. Comera, X. Collet, F. Kuipers, M. Krempf, B. Cariou, P. Costet, Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia. Arterioscler. Thromb. Vasc. Biol. 29(5), 684–690 (2009)
C. Le May, J.M. Berger, A. Lespine, B. Pillot, X. Prieur, E. Letessier, M.M. Hussain, X. Collet, B. Cariou, P. Costet, Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 33(7), 1484–1493 (2013). doi:10.1161/ATVBAHA.112.300263
G. Paradis, M. Lambert, J. O’Loughlin, C. Lavallee, J. Aubin, P. Berthiaume, M. Ledoux, E.E. Delvin, E. Levy, J.A. Hanley, The Quebec Child and Adolescent Health and Social Survey: design and methods of a cardiovascular risk factor survey for youth. Can. J. Cardiol. 19(5), 523–531 (2003)
R.G. Victor, R.W. Haley, D.L. Willett, R.M. Peshock, P.C. Vaeth, D. Leonard, M. Basit, R.S. Cooper, V.G. Iannacchione, W.A. Visscher, J.M. Staab, H.H. Hobbs, Dallas Heart Study Investigators, The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am. J. Cardiol. 93(12), 1473–1480 (2004). doi:10.1016/j.amjcard.2004.02.058
M. Abifadel, M. Guerin, S. Benjannet, J.P. Rabes, W. Le Goff, Z. Julia, J. Hamelin, V. Carreau, M. Varret, E. Bruckert, L. Tosolini, O. Meilhac, P. Couvert, D. Bonnefont-Rousselot, J. Chapman, A. Carrie, J.B. Michel, A. Prat, N.G. Seidah, C. Boileau, Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia. Atherosclerosis 223(2), 394–400 (2012). doi:10.1016/j.atherosclerosis.2012.04.006
L.H. Aung, R.X. Yin, L. Miao, X.J. Hu, T.T. Yan, X.L. Cao, D.F. Wu, Q. Li, S.L. Pan, J.Z. Wu, The proprotein convertase subtilisin/kexin type 9 gene E670G polymorphism and serum lipid levels in the Guangxi Bai Ku Yao and Han populations. Lipids Health Dis. 10, 5 (2011). doi:10.1186/1476-511X-10-5
S. Hirayama, T. Miida, Small dense LDL: an emerging risk factor for cardiovascular disease. Clin. Chim. Acta 414, 215–224 (2012). doi:10.1016/j.cca.2012.09.010
S. Koba, Y. Yokota, T. Hirano, Y. Ito, Y. Ban, F. Tsunoda, T. Sato, M. Shoji, H. Suzuki, E. Geshi, Y. Kobayashi, T. Katagiri, Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J. Atheroscler. Thromb. 15(5), 250–260 (2008)
M.R. Diffenderfer, E.J. Schaefer, The composition and metabolism of large and small LDL. Curr. Opin. Lipidol. 25(3), 221–226 (2014). doi:10.1097/MOL.0000000000000067
Y. Zhang, R.X. Xu, S. Li, C.G. Zhu, Y.L. Guo, J. Sun, J.J. Li, Association of plasma small dense LDL cholesterol with PCSK9 levels in patients with angiographically proven coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 25(4), 426–433 (2015). doi:10.1016/j.numecd.2015.01.006
C. Zheng, C. Khoo, J. Furtado, F.M. Sacks, Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 121(15), 1722–1734 (2010). doi:10.1161/CIRCULATIONAHA.109.875807
M. Abifadel, M. Varret, J.P. Rabes, D. Allard, K. Ouguerram, M. Devillers, C. Cruaud, S. Benjannet, L. Wickham, D. Erlich, A. Derre, L. Villeger, M. Farnier, I. Beucler, E. Bruckert, J. Chambaz, B. Chanu, J.M. Lecerf, G. Luc, P. Moulin, J. Weissenbach, A. Prat, M. Krempf, C. Junien, N.G. Seidah, C. Boileau, Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34(2), 154–156 (2003)
Acknowledgments
Fondazione Cariplo Grants Rif. 2012-0549 (N.F.) and Rif. 2015-0552 (M.R.) and Piano di Sostegno per la Ricerca, Università degli Studi di Milano, 2015-2017 - Linea 2 (Azione A) (M.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All the authors declare that they have no conflict of interest with the contents of this article.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s12020-016-1109-0.
Rights and permissions
About this article
Cite this article
Ferri, N., Ruscica, M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome: insights on insulin resistance, inflammation, and atherogenic dyslipidemia. Endocrine 54, 588–601 (2016). https://doi.org/10.1007/s12020-016-0939-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0939-0