Abstract
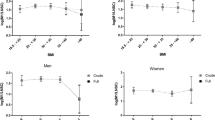
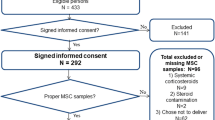
Cross-sectional association has been shown between type 2 diabetes and hypothalamic–pituitary–adrenal (HPA) axis dysregulation; however, the temporality of this association is unknown. Our aim was to determine if type 2 diabetes is associated with longitudinal change in daily cortisol curve features. We hypothesized that the presence of type 2 diabetes may lead to a more blunted and abnormal HPA axis profile over time, suggestive of increased HPA axis dysregulation. This was a longitudinal cohort study, including 580 community-dwelling individuals (mean age 63.7 ± 9.1 years; 52.8 % women) with (n = 90) and without (n = 490) type 2 diabetes who attended two MultiEthnic Study of Atherosclerosis Stress ancillary study exams separated by 6 years. Outcome measures that were collected were wake-up and bedtime cortisol, cortisol awakening response (CAR), total area under the curve (AUC), and early, late, and overall decline slopes. In univariate analyses, wake-up and AUC increased over 6 years more in persons with as compared to those without type 2 diabetes (11 vs. 7 % increase for wake-up and 17 vs. 11 % for AUC). The early decline slope became flatter over time with a greater flattening observed in diabetic compared to non-diabetic individuals (23 vs. 9 % flatter); however, the change was only statistically significant for wake-up cortisol (p-value: 0.03). Over time, while CAR was reduced more, late decline and overall decline became flatter, and bedtime cortisol increased less in those with as compared to those without type 2 diabetes, none of these changes were statistically significant in adjusted models. We did not identify any statistically significant change in cortisol curve features over 6 years by type 2 diabetes status.


Similar content being viewed by others
References
P.M. Peeke, G.P. Chrousos, Hypercortisolism and obesity. Ann. N. Y. Acad. Sci. 771, 665–676 (1995)
G. Mazziotti, C. Gazzaruso, A. Giustina, Diabetes in cushing syndrome: basic and clinical aspects. TEM 22(12), 499–506 (2011). doi:10.1016/j.tem.2011.09.001
P. Anagnostis, V.G. Athyros, K. Tziomalos, A. Karagiannis, D.P. Mikhailidis, Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J. Clin. Endocrinol. Metab. 94(8), 2692–2701 (2009). doi:10.1210/jc.2009-0370
H. Bruehl, M. Rueger, I. Dziobek, V. Sweat, A. Tirsi, E. Javier, A. Arentoft, O.T. Wolf, A. Convit, Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J. Clin. Endocrinol. Metab. 92(7), 2439–2445 (2007). doi:10.1210/jc.2006-2540
I. Chiodini, M. Torlontano, A. Scillitani, M. Arosio, S. Bacci, S. Di Lembo, P. Epaminonda, G. Augello, R. Enrini, B. Ambrosi, G. Adda, V. Trischitta, Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. Eur. J. Endocrinol. 153(6), 837–844 (2005). doi:10.1530/eje.1.02045
A.F. Godoy-Matos, A.R. Vieira, R.O. Moreira, W.F. Coutinho, L.M. Carraro, D.M. Moreira, R. Pasquali, R.M. Meirelles, The potential role of increased adrenal volume in the pathophysiology of obesity-related type 2 diabetes. J. Endocrinol. Invest. 29(2), 159–163 (2006)
S. Champaneri, X. Xu, M.R. Carnethon, A.G. Bertoni, T. Seeman, A.S. DeSantis, A. Diez Roux, S. Shrager, S.H. Golden, Diurnal salivary cortisol is associated with body mass index and waist circumference: the multiethnic study of atherosclerosis. Obesity 21(1), E56–E63 (2013). doi:10.1002/oby.20047
R. Rosmond, S. Wallerius, P. Wanger, L. Martin, G. Holm, P. Bjorntorp, A 5-year follow-up study of disease incidence in men with an abnormal hormone pattern. J. Intern. Med. 254(4), 386–390 (2003)
A. Gungunes, M. Sahin, T. Demirci, B. Ucan, E. Cakir, M.S. Arslan, I.O. Unsal, B. Karbek, M. Caliskan, M. Ozbek, E. Cakal, T. Delibasi, Cushing’s syndrome in type 2 diabetes patients with poor glycemic control. Endocrine 47(3), 895–900 (2014). doi:10.1007/s12020-014-0260-8
B. Catargi, V. Rigalleau, A. Poussin, N. Ronci-Chaix, V. Bex, V. Vergnot, H. Gin, P. Roger, A. Tabarin, Occult Cushing’s syndrome in type-2 diabetes. J. Clin. Endocrinol. Metab. 88(12), 5808–5813 (2003). doi:10.1210/jc.2003-030254
L. Wei, T.M. MacDonald, B.R. Walker, Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann. Intern. Med. 141(10), 764–770 (2004)
P.C. Souverein, A. Berard, T.P. Van Staa, C. Cooper, A.C. Egberts, H.G. Leufkens, B.R. Walker, Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 90(8), 859–865 (2004). doi:10.1136/hrt.2003.020180
J. Etxabe, J.A. Vazquez, Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin. Endocrinol. 40(4), 479–484 (1994)
N.M. Neary, O.J. Booker, B.S. Abel, J.R. Matta, N. Muldoon, N. Sinaii, R.I. Pettigrew, L.K. Nieman, A.M. Gharib, Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J. Clin. Endocrinol. Metab. 98(5), 2045–2052 (2013). doi:10.1210/jc.2012-3754
S. Champaneri, G.S. Wand, S.S. Malhotra, S.S. Casagrande, S.H. Golden, Biological basis of depression in adults with diabetes. Curr. Diab. Rep. 10(6), 396–405 (2010). doi:10.1007/s11892-010-0148-9
P.E. Szmitko, C.H. Wang, R.D. Weisel, J.R. de Almeida, T.J. Anderson, S. Verma, New markers of inflammation and endothelial cell activation: part I. Circulation 108(16), 1917–1923 (2003). doi:10.1161/01.CIR.0000089190.95415.9F
P.M. Ridker, J.E. Buring, J. Shih, M. Matias, C.H. Hennekens, Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 98(8), 731–733 (1998)
J.C. Pruessner, O.T. Wolf, D.H. Hellhammer, A. Buske-Kirschbaum, K. von Auer, S. Jobst, F. Kaspers, C. Kirschbaum, Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 61(26), 2539–2549 (1997)
C. Kirschbaum, D.H. Hellhammer, Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22(3), 150–169 (1989).
A.S. Karlamangla, E.M. Friedman, T.E. Seeman, R.S. Stawksi, D.M. Almeida, Daytime trajectories of cortisol: demographic and socioeconomic differences—findings from the National Study of Daily Experiences. Psychoneuroendocrinology 38(11), 2585–2597 (2013). doi:10.1016/j.psyneuen.2013.06.010
S.E. Sephton, R.M. Sapolsky, H.C. Kraemer, D. Spiegel, Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 92(12), 994–1000 (2000)
E.K. Adam, M.R. Gunnar, Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology 26(2), 189–208 (2001)
M.R. Gunnar, D.M. Vazquez, Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 13(3), 515–538 (2001)
S.R. Kunz-Ebrecht, C. Kirschbaum, M. Marmot, A. Steptoe, Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology 29(4), 516–528 (2004)
C.E. Wright, A. Steptoe, Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology 30(6), 582–590 (2005). doi:10.1016/j.psyneuen.2005.01.007
S. Cohen, J.E. Schwartz, E. Epel, C. Kirschbaum, S. Sidney, T. Seeman, Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med. 68(1), 41–50 (2006). doi:10.1097/01.psy.0000195967.51768.ea
D.E. Saxbe, R.L. Repetti, A. Nishina, Marital satisfaction, recovery from work, and diurnal cortisol among men and women. Health Psychol. 27(1), 15–25 (2008). doi:10.1037/0278-6133.27.1.15
A. Hajat, A. Diez-Roux, T.G. Franklin, T. Seeman, S. Shrager, N. Ranjit, C. Castro, K. Watson, B. Sanchez, C. Kirschbaum, Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 35(6), 932–943 (2010). doi:10.1016/j.psyneuen.2009.12.009
M. Kumari, M. Shipley, M. Stafford, M. Kivimaki, Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. metab. 96(5), 1478–1485 (2011). doi:10.1210/jc.2010-2137
X. Wang, B.N. Sanchez, S.H. Golden, S. Shrager, C. Kirschbaum, A.S. Karlamangla, T.E. Seeman, A.V. Roux, Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology 49, 310–320 (2014). doi:10.1016/j.psyneuen.2014.07.024
S.H. Golden, B.N. Sanchez, M. Wu, S. Champaneri, A.V. Diez Roux, T. Seeman, G.S. Wand, Relationship between the cortisol awakening response and other features of the diurnal cortisol rhythm: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 38(11), 2720–2728 (2013). doi:10.1016/j.psyneuen.2013.06.032
S.H. Golden, G.S. Wand, S. Malhotra, I. Kamel, K. Horton, Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. Eur. J. Epidemiol. 26(7), 511–525 (2011). doi:10.1007/s10654-011-9585-2
G. Tirabassi, M. Boscaro, G. Arnaldi, Harmful effects of functional hypercortisolism: a working hypothesis. Endocrine 46(3), 370–386 (2014). doi:10.1007/s12020-013-0112-y
J.J. Joseph, X. Wang, E. Spanakis, T. Seeman, G. Wand, B. Needham, S.H. Golden, Diurnal salivary cortisol, glycemia and insulin resistance: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 62, 327–335 (2015). doi:10.1016/j.psyneuen.2015.08.021
D.E. Bild, D.A. Bluemke, G.L. Burke, R. Detrano, A.V. Diez Roux, A.R. Folsom, P. Greenland, D.R. Jacob Jr, R. Kronmal, K. Liu, J.C. Nelson, D. O’Leary, M.F. Saad, S. Shea, M. Szklo, R.P. Tracy, Multi-ethnic study of atherosclerosis: objectives and design. Am. J. Epidemiol. 156(9), 871–881 (2002)
H. Raff, Update on late-night salivary cortisol for the diagnosis of Cushing’s syndrome: methodological considerations. Endocrine 44(2), 346–349 (2013). doi:10.1007/s12020-013-0013-0
G. Bellastella, M.I. Maiorino, A. De Bellis, M.T. Vietri, C. Mosca, L. Scappaticcio, D. Pasquali, K. Esposito, D. Giugliano, Serum but not salivary cortisol levels are influenced by daily glycemic oscillations in type 2 diabetes. Endocrine (2015). doi:10.1007/s12020-015-0777-5
S. Hill Golden, B.N. Sanchez, A.S. Desantis, M. Wu, C. Castro, T.E. Seeman, S. Tadros, S. Shrager, A.V. Diez Roux, Salivary cortisol protocol adherence and reliability by socio-demographic features: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 43, 30–40 (2014). doi:10.1016/j.psyneuen.2014.01.025
D.H. Hellhammer, S. Wust, B.M. Kudielka, Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34(2), 163–171 (2009). doi:10.1016/j.psyneuen.2008.10.026
K.C. Yeh, K.C. Kwan, A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J. Pharmacokinet. Biopharm. 6(1), 79–98 (1978)
E. Badrick, C. Kirschbaum, M. Kumari, The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. metab. 92(3), 819–824 (2007). doi:10.1210/jc.2006-2155
A.G. Bertoni, M.C. Whitt-Glover, H. Chung, K.Y. Le, R.G. Barr, M. Mahesh, N.S. Jenny, G.L. Burke, D.R. Jacobs, The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 169(4), 444–454 (2009). doi:10.1093/aje/kwn350
L. Radloff, The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401 (1977)
P.A. Pilkonis, S.D. Imber, P. Rubinsky, Dimensions of life stress in psychiatric patients. J. Human Stress 11(1), 5–10 (1985). doi:10.1080/0097840X.1985.9936732
M.S. Mujahid, A.V. Diez Roux, R.C. Cooper, S. Shea, D.R. Williams, Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am. J. Hypertens. 24(2), 187–193 (2011). doi:10.1038/ajh.2010.200
E.K. Adam, M. Kumari, Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34(10), 1423–1436 (2009). doi:10.1016/j.psyneuen.2009.06.011
S. Champaneri, X. Xu, M.R. Carnethon, A.G. Bertoni, T. Seeman, A. Diez Roux, S.H. Golden, Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism 61(7), 986–995 (2012). doi:10.1016/j.metabol.2011.11.006
G. Casella, R. Berger, Statistical Inference, vol. 2 (Duxbury Press, Pacific Grove, 2001)
H. Bruehl, O.T. Wolf, A. Convit, A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 34(6), 815–821 (2009). doi:10.1016/j.psyneuen.2008.12.010
F. Lederbogen, J. Hummel, C. Fademrecht, B. Krumm, C. Kuhner, M. Deuschle, K.H. Ladwig, C. Meisinger, H.E. Wichmann, H. Lutz, B. Breivogel, Flattened circadian cortisol rhythm in type 2 diabetes. Exp. Clin. Endocrinol. Diabet. 119(9), 573–575 (2011). doi:10.1055/s-0031-1275288
S.A. Vreeburg, B.P. Kruijtzer, J. van Pelt, R. van Dyck, R.H. DeRijk, W.J. Hoogendijk, J.H. Smit, F.G. Zitman, B.W. Penninx, Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology 34(8), 1109–1120 (2009). doi:10.1016/j.psyneuen.2009.04.024
R.A. Hackett, A. Steptoe, M. Kumari, Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J. Clin. Endocrinol. metab. (2014). doi:10.1210/jc.2014-2459
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
MESA was supported by contracts NO1-HC-95159 through NO1-HC-95165 and NO1-HC-95169 from the National Heart, Lung, and Blood Institute (PI: ADR). MESA Stress Study was supported by RO1 HL10161-01A1 and R21 DA024273 (PI: ADR). EKS was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32DK062707).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant conflict of interest to disclose.
Ethical Standards
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national committees and have been performed in accordance with the ethical standards as laid down in the 1964 Helsinki declaration and its latter amendments or comparable ethical standards.
Informed Consent
All participants provided informed consent and the above studies were approved by the Institutional Review Boards of each institution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Appendix 1
Let \( y_{ijdk} \) be the kth measure of cortisol of subject \( i \) at MESA Stress study \( j \) (\( j \) = 1, 2) on day \( d \). The piecewise linear mixed effect model was specified as follows:
where \( \beta_{li} = \beta_{l} + b_{li} , l = 0, 1, 3, 4,5,7 \) and \( b_{0i} , b_{1i} ,b_{3i} ,b_{4i} , b_{5i} ,b_{7i} \) are individual-level random intercept and slope for individual \( i \);
\( {\text{Time}}_{ij} \) is the time (years) since the baseline study (MESA Stress I) for individual \( i \) at study \( j \). Note that \( {\text{Time}}_{{{\text{i}}1}} = 0 \); \( t_{ijdk} \) is the time (h) since wake-up when the cortisol sample \( y_{ijdk} \) was collected;
\( {\text{Cov}}_{i} \) represents a set of sociodemographic factors and health-related factors for individual \( i \) at baseline study; \( {\text{Cov}}_{i} \) is excluded in Model 0 as the model is for an unadjusted analysis; \( {\text{Cov}}_{i} \) includes sociodemographic factors including age, sex, race/ethnicity, and socioeconomic status in Model 1; and additionally includes waist circumference, depressive symptoms, smoking status, and medication usage (beta-blocker, aspirins, inhaled or oral steroids, and hormone replacement therapy) in Model 2. Also, all covariates included in \( {\text{Cov}}_{i} \) are centered at their population average in the analysis; therefore, the estimates on the difference in cortisol feature change over time for each diabetes groups and the difference between groups (as shown in Supplementary Table 1) are interpreted at the population average, i.e., average level of sociodemographic characteristics and health-related factors.
\( {\text{Diab}}_{i} \) is a binary variable indicating individual’s diabetes status (1: diabetes; 0: non-diabetes); \( e_{ijdk} \) is the unexplained deviation from the mean for the kth cortisol measure on day d at MESA Stress study j for individual i.
The estimates of the coefficients for the terms that involve diabetes status were used to derive estimates of the cortisol features by diabetes groups, and the difference in the change of daily cortisol features over time between diabetes groups, as shown in Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Spanakis, E.K., Wang, X., Sánchez, B.N. et al. Lack of significant association between type 2 diabetes mellitus with longitudinal change in diurnal salivary cortisol: the multiethnic study of atherosclerosis. Endocrine 53, 227–239 (2016). https://doi.org/10.1007/s12020-016-0887-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0887-8