Abstract
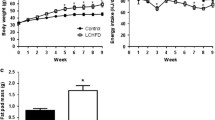
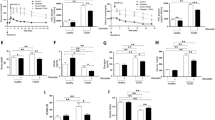
Previous studies suggested that endogenous glucagon-like peptide 2 (GLP-2) is dispensable for the regulation of glucose homeostasis under normal conditions, while it can play a beneficial role in obesity conditions. The purpose of the present study was to investigate whether chronic treatment with Gly2-GLP-2, a stable analogue of GLP-2, can have an impact on glycaemic and lipid control in mice fed a high-fat diet (HFD), an animal model of human obesity and insulin resistance. HFD mice were treated once a day with Gly2-GLP-2 for 4 weeks. Body weight, food intake, fasting glucose, intraperitoneal glucose tolerance, insulin-induced glucose clearance, glucose-stimulated insulin secretion, β-cell mass, plasma lipid metabolic profile, and lipid deposition in the liver were examined. In untreated HFD mice, fasting glucose levels, glucose tolerance, glucose-stimulated plasma insulin and sensibility to exogenous insulin were deteriorating with time and β-cell mass increased. In Gly2-GLP-2-treated mice, we found significant increase in glucose tolerance and exogenous insulin sensitivity, reduction in glucose-stimulated plasma insulin and in the increase in β-cell mass in comparison with pair-aged HFD untreated animals. The chronic treatment with the peptide was not associated with remarkable improvements of dyslipidemia and it did not prevent liver fat accumulation and the presence of microvesicular steatosis. In conclusion, the results of the present study suggest, for the first time, that Gly2-GLP-2 may produce glucose metabolic benefits in mice with diet-induced obesity. The mechanisms underlying the beneficial impact of GLP-2 on glucose metabolism remain to be established.





Similar content being viewed by others
References
A.S. Al-Goblan, M.A. Al-Alfi, M.Z. Khan, Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 7, 587–591 (2014)
I. Vaněčková, L. Maletínská, M. Behuliak, V. Nagelová, J. Zicha, J. Kuneš, Obesity-related hypertension: possible pathophysiological mechanisms. J. Endocrinol. 223, R63–R78 (2014)
M.K. Badman, J.S. Flier, The gut and energy balance: visceral allies in the obesity wars. Science 307, 1909–1914 (2005)
S. Baldassano, A. Amato, GLP-2: what do we know? What are we going to discover? Regul. Pept. 194–195, 6–10 (2014)
K.J. Rowland, P.L. Brubaker, The “cryptic” mechanism of action of glucagon-like peptide-2. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G1–G8 (2011)
P. Janssen, A. Rotondo, F. Mulé, J. Tack, Review article: a comparison of glucagon-like peptides 1 and 2. Aliment. Pharmacol. Ther. 37, 18–36 (2013)
K. Vipperla, S.J. O’Keefe, Targeted therapy of short-bowel syndrome with teduglutide: the new kid on the block. Clin. Exp. Gastroenterol. 7, 489–495 (2014)
J.F. Marier, M.S. Mouksassi, N.H. Gosselin, M. Beliveau, J. Cyran, J. Wallens, Population pharmacokinetics of teduglutide following repeated subcutaneous administrations in healthy participants and in patients with short bowel syndrome and Crohn’s disease. J. Clin. Pharmacol. 50, 36–49 (2010)
H. Kissow, Glucagon-like peptides 1 and 2: intestinal hormones implicated in the pathophysiology of mucositis. Curr. Opin. Support Palliat. Care 9, 196–202 (2015)
M. Wøjdemann, A. Wettergren, B. Hartmann, J.J. Holst, Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand. J. Gastroenterol. 33, 828–832 (1998)
A. Amato, S. Baldassano, R. Serio, F. Mulè, Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G678–G684 (2009)
A. Amato, A. Rotondo, L. Cinci, S. Baldassano, M.G. Vannucchi, F. Mulè, Role of cholinergic neurons in the motor effects of glucagon-like peptide-2 in mouse colon. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1038–G1044 (2010)
L. Cinci, M.S. Faussone-Pellegrini, A. Rotondo, F. Mulè, M.G. Vannucchi, GLP-2 receptor expression in excitatory and inhibitory enteric neurons and its role in mouse duodenum contractility. Neurogastroenterol. Motil. 23, e383–e392 (2011)
S. Baldassano, S. Liu, M.H. Qu, F. Mulè, J.D. Wood, Glucagon-like peptide-2 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G800–G805 (2009)
A. Rotondo, A. Amato, S. Baldassano, L. Lentini, F. Mulè, Gastric relaxation induced by glucagon-like peptide-2 in mice fed a high-fat diet or fasted. Peptides 32, 1587–1592 (2011)
S. Baldassano, A.L. Bellanca, R. Serio, F. Mulè, Food intake in lean and obese mice after peripheral administration of glucagon-like peptide 2. J. Endocrinol. 213, 277–284 (2012)
M. Tang-Christensen, P.J. Larsen, J. Thulesen, J. Romer, N. Vrang, The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat. Med. 6, 802–807 (2000)
X. Shi, F. Zhou, X. Li, B. Chang, D. Li, Y. Wang, Q. Tong, Y. Xu, M. Fukuda, J.J. Zhao, D. Li, D.G. Burrin, L. Chan, X. Guan, Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab. 18, 86–98 (2013)
X. Guan, The CNS glucagon-like peptide-2 receptor in the control of energy balance and glucose homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R585–R596 (2014)
J. Hsieh, C. Longuet, A. Maida, J. Bahrami, E. Xu, C.L. Baker, P.L. Brubaker, D.J. Drucker, K. Adeli, Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 137, 997–1005 (2009)
G.F. Watts, D.C. Chan, Novel insights into the regulation of postprandial lipemia by glucagon-like peptides: significance for diabetes. Diabetes 62, 336–338 (2013)
S. Dash, C. Xiao, C. Morgantini, P.W. Connelly, B.W. Patterson, G.F. Lewis, Glucagon-like peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology 147, 1275–1284 (2014)
P.D. Cani, S. Possemiers, T. Van de Wiele, Y. Guiot, A. Everard, O. Rottier, L. Geurts, D. Naslain, A. Neyrinck, D.M. Lambert, G.G. Muccioli, N.M. Delzenne, Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009)
J. Bahrami, C. Longuet, L.L. Baggio, K. Li, D.J. Drucker, Glucagon-like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob mouse. Gastroenterology 139, 857–868 (2010)
S. Baldassano, F. Rappa, A. Amato, F. Cappello, F. Mulè, GLP-2 as beneficial factor in the glucose homeostasis in mice fed a high fat diet. J. Cell. Physiol. 230, 3029–3036 (2015)
I. Hadjiyanni, K.K. Li, D.J. Drucker, Glucagon-like peptide-2 reduces intestinal permeability but does not modify the onset of type 1 diabetes in the nonobese diabetic mouse. Endocrinology 150, 529–592 (2009)
R. Iakoubov, L.M. Lauffer, S. Trivedi, Y.I. Kim, P.L. Brubaker, Carcinogenic effects of exogenous and endogenous glucagon-like peptide-2 in azoxymethane-treated mice. Endocrinology 150, 4033–4043 (2009)
J. Folch, M. Lees, G.H. Sloane Stanley, A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957)
A.M. Johnson, J.M. Olefsky, The origins and drives of insulin resistance. Cell 152, 673–684 (2013)
L. Prasad-Reddy, D. Isaacs, A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 4, 212283 (2015)
L.L. Baggio, D.J. Drucker, Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007)
A.M. Deane, N.Q. Nguyen, J.E. Stevens, R.J.L. Fraser, R.H. Holloway, L.K. Besanko, C. Burgstad, K.L. Jones, M.J. Chapman, C.K. Rayner, M. Horowitz, Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J. Clin. Endocrinol. Metabol. 95, 215–221 (2010)
A. Rotondo, A. Amato, L. Lentini, S. Baldassano, F. Mulè, Glucagon-like peptide-1 relaxes gastric antrum through nitric oxide in mice. Peptides 32, 60–64 (2011)
J. Hsieh, C. Longuet, C.L. Baker, B. Qin, L.M. Federico, D.J. Drucker, K. Adeli, The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 53, 552–561 (2010)
J.P. Gutzwiller, B. Goke, J. Drewe, P. Hildebrand, S. Ketterer, D. Handschin, R. Winterhalder, D. Conen, C. Beglinger, Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44, 81–86 (1999)
A. Amato, L. Cinci, A. Rotondo, R. Serio, M.S. Faussone-Pellegrini, M.G. Vannucchi, F. Mulè, Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol. Motil. 22, 664–e203 (2010)
A. Amato, S. Baldassano, R. Liotta, R. Serio, F. Mulè, Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle. J. Endocrinol. 221, 29–37 (2014)
S. Baldassano, G.D. Wang, F. Mulè, J.D. Wood, Glucagon-like peptide-1 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G352–G358 (2012)
C.Y. Shan, J.H. Yang, Y. Kong, X.Y. Wang, M.Y. Zheng, Y.G. Xu, Y. Wang, H.Z. Ren, B.C. Chang, L.M. Chen, Alteration of the intestinal barrier and GLP2 secretion in Berberine-treated type 2 diabetic rats. J. Endocrinol. 218, 255–262 (2013)
R.S. Surwit, C.M. Kuhn, C. Cochrane, J.A. McCubbin, M.N. Feinglos, Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37, 1163–1167 (1988)
M.S. Winzell, B. Ahren, The high-fat diet–fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, S215–S219 (2004)
M. He, H. Su, W. Gao, S.M. Johansson, Q. Liu, X. Wu, J. Liao, A.A. Young, T. Bartfai, M.W. Wang, Reversal of obesity and insulin resistance by a non-peptidic glucagon-like peptide-1 receptor agonist in diet-induced obese mice. PLoS One 5, e14205 (2010)
R.E. Stamateris, R.B. Sharma, D.A. Hollern, L.C. Alonso, Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am. J. Physiol. Endocrinol. Metab. 305, E149–E159 (2013)
A.E. Butler, J. Janson, S. Bonner-Weir, R. Ritzel, R.A. Rizza, P.C. Butler, Beta cell deficit and increased beta cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003)
J. Ahren, B. Ahren, N. Wierup, Increased beta cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets 2, 353–356 (2010)
B.A. Omar, J. Vikman, M.S. Winzell, U. Voss, E. Ekblad, J.E. Foley, B. Ahrén, Enhanced beta cell function and anti-inflammatory effect after chronic treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin in an advanced-aged diet-induced obesity mouse model. Diabetologia 56, 1752–1760 (2013)
T. Okada, C.W. Liew, J. Hu, C. Hinault, M.D. Michael, J. Krtzfeldt, C. Yin, M. Holzenberger, M. Stoffel, R.N. Kulkarni, Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc. Natl. Acad. Sci. USA. 104, 8977–8982 (2007)
M.M. Sachdeva, D.A. Stoffers, Minireview: meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol. Endocrinol. 23, 747–758 (2009)
B.R. Gedulin, S.E. Nikoulina, P.A. Smith, G. Gedulin, L.L. Nielsen, A.D. Baron, D.G. Parkes, A.A. Young, Exenatide (exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 146, 2069–2076 (2005)
Y. Matsuzawa, T. Funahashi, T. Nakamura, Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann. NY Acad. Sci. 892, 146–154 (1999)
M.W. Rajala, S. Obici, P.E. Scherer, L. Rossetti, Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J. Clin. Invest. 111, 225–230 (2003)
H. Yki-Järvinen, J. Westerbacka, The fatty liver and insulin resistance. Curr. Mol. Med. 5, 287–295 (2005)
M. Asrih, F.R. Jornayvaz, Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J. Endocrinol. 218, R25–R36 (2013)
V.E. de Meijer, H.D. Le, J.A. Meisel, M.R. Akhavan Sharif, A. Pan, V. Nosé, M. Puder, Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism 59, 1092–10105 (2010)
J.C. Fraulob, R. Ogg-Diamantino, C. Fernandes-Santos, M.B. Aguila, C.A. Mandarim-de-Lacerda, A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J. Clin. Biochem. Nutr. 46, 212–223 (2010)
N. El-Jamal, E. Erdual, M. Neunlist, D. Koriche, C. Dubuquoy, F. Maggiotto, J. Chevalier, D. Berrebi, L. Dubuquoy, E. Boulanger, A. Cortot, P. Desreumaux, Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G274–G285 (2014)
B.A. Moore, N. Peffer, A. Pirone, A. Bassiri, S. Sague, J.M. Palmer, D.L. Johnson, T. Nesspor, C. Kliwinski, P.J. Hornby, GLP-2 receptor agonism ameliorates inflammation and gastrointestinal stasis in murine postoperative ileus. J. Pharmacol. Exp. Ther. 333, 574–583 (2010)
G.W. Moran, C. O’Neill, J.T. McLaughlin, GLP-2 enhances barrier formation and attenuates TNFα-induced changes in a Caco-2 cell model of the intestinal barrier. Regul. Pept. 178, 95–101 (2012)
S. Baldassano, A. Amato, F. Cappello, F. Rappa, F. Mulè, Glucagon-like peptide-2 and mouse intestinal adaptation to a high-fat diet. J. Endocrinol. 217, 11–20 (2013)
Acknowledgments
This work was supported by a Grant from Ministero dell’Istruzione, dell’Università e della Ricerca, Italy (FFR 2012, University of Palermo).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baldassano, S., Amato, A., Caldara, G.F. et al. Glucagon-like peptide-2 treatment improves glucose dysmetabolism in mice fed a high-fat diet. Endocrine 54, 648–656 (2016). https://doi.org/10.1007/s12020-016-0871-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0871-3