Abstract
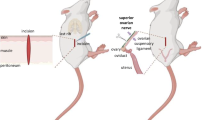
In vitro the vasoactive intestinal peptide (VIP) stimulates progesterone, androgens, and estradiol secretion, and the effects are time-dependent. The present study analyzed the acute (1 h) and sub-acute (24 h) effects of unilateral injection of VIP into the ovarian bursa on each day of the estrous cycle on progesterone, testosterone, and estradiol serum levels. Cyclic 60-day-old virgin female rats on diestrus-1, diestrus-2, proestrus, or estrus were injected with saline or VIP 10−6 M into the left or right ovarian bursa. One hour after saline injection on each day of estrus cycle, progesterone levels were higher than in control animals. The acute effects of saline solution on testosterone and estradiol levels were asymmetric and varied during the estrous cycle. In comparison with saline groups, the effects of VIPergic stimulation on progesterone, testosterone, and estradiol serum levels depend on the time elapsed between treatment and autopsy and vary during the estrous cycle. An acute asymmetric response from the ovaries to the VIP was observed at diestrus-1, diestrus-2, and proestrus on progesterone and estradiol levels. The asymmetries on testosterone levels were observed at diestrus-1, diestrus-2, and estrus days. The present results suggest that in the cyclic rat, each ovary has different sensitivities to VIPergic stimulation which depends on the endocrine status of the animal.



Similar content being viewed by others
References
M.L. Forneris, L.I. Aguado, Neonatal superior ovarian nerve transection disturbs the cyclic activity of the female rats. J. Steroid Biochem. Mol. Biol. 82, 75–82 (2002)
F. Kagitani, S. Uchida, H. Hotta, Effects of electrical stimulation of the superior ovarian nerve and the ovarian plexus nerve on the ovarian estradiol secretion rate in rats. J Physiol Sci. 58, 133–138 (2008)
R. Domínguez, A. Flores, S.E. Cruz-Morales, in Hormonal and Neural Mechanisms Regulating Hormone Steroids Secretion, ed. by A. Hassan. Steroids Basic Science, Chaper 1, 1st edn. (In Tech, Croacia, Rijeka, 2011), pp. 3–34. ISBN 978-953-307-866-3 (2011)
W.L. Miller, R.J. Auchus, The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011)
L. Morales-Ledesma, D.A. Ramírez, E. Vieyra, A. Trujillo, R. Chavira, M. Cárdenas, R. Domínguez, Effects of acute unilateral ovariectomy to pre-pubertal rats on steroid hormones secretion and compensatory ovarian responses. Reprod. Biol. Endocrinol. 9, 41 (2011)
L. Morales-Ledesma, E. Vieyra, D.A. Ramírez, A. Trujillo, R. Chavira, M. Cárdenas, R. Domínguez, Effects on steroid hormones secretion resulting from the acute stimulation of sectioning the superior ovarian nerve to pre-pubertal rats. Reprod. Biol. Endocrinol. 10, 88 (2012)
I.E. Lawrence Jr, H.W. Burden, The origin of the extrinsic adrenergic innervation to the rat ovary. Anat. Rec. 196, 51–59 (1980)
B. Baljet, J. Drukker, The extrinsic innervation of the abdominal organs in the female rat. Acta Anat. (Basel) 104, 243–267 (1979)
J.M. Bahr, N. Ben-Jonathan, Preovulatory depletion of ovarian catecholamines in the rat. Endocrinology 108, 1815–1820 (1981)
L.I. Aguado, S.R. Ojeda, Prepubertal ovarian function is finely regulated by direct adrenergic influences. Role of noradrenergic innervation. Endocrinology. 114, 1845–1853 (1984)
W.L. Dees, C.E. Ahmed, S.R. Ojeda, Substance P- and vasoactive intestinal peptide-containing fibers reach the ovary by independent routes. Endocrinology 119, 638–641 (1986)
S.I. Said, R.N. Rosenberg, Vasoactive intestinal peptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science 192, 907–908 (1976)
S.I. Said, V. Mutt, Polypeptide with broad biological activity: isolation from small Intestine. Science 169, 1217–1218 (1970)
I. Gozes, A. Tsafriri, Detection of vasoactive intestinal peptide-encoding messenger ribonucleic acid in the rat ovaries. Endocrinology 119, 2606–2610 (1986)
S.C.J. Hulshof, G. Dijkstra, E.M. Van Der Beek, M.M. Bevers, J.R. Figueiredo, J.F. Beckers, R. Van Den Hurk, Immunocytochemical localization of vasoactive intestinal peptide and neuropeptide Y in the bovine ovary. Biol. Reprod. 50, 553–560 (1994)
C.E. Ahmed, W.L. Dees, S.R. Ojeda, The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology 118, 1682–1689 (1986)
S. Onoue, S. Misaka, S. Yamada, Structure-activity relationship of vasoactive intestinal peptide (VIP): potent agonists and potential clinical applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 377, 579–590 (2008)
S. Vaccari, S. Latini, M. Barberi, A. Teti, M. Stefanini, R. Canipari, Characterization and expression of different pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptors in rat ovarian follicles. J. Endocrinol. 191, 287–299 (2006)
A. Mayerhofer, G.A. Dissen, M.E. Costa, S.R. Ojeda, A role for neurotransmitters in early follicular development: induction of functional follicle-stimulating hormone receptors in newly formed follicles of the rat ovary. Endocrinology 138, 3320–3329 (1997)
N. Chen, Y. Li, W. Wang, Y. Ma, D. Yang, Q. Zhang, Vasoactive intestinal peptide can promote the development of neonatal rat primordial follicles during in vitro culture. Biol. Reprod. 88, 12 (2013)
J. Törnell, B. Carlsson, T. Hillensjö, Vasoactive intestinal peptide stimulates oocyte maturation, steroidogenesis, and cyclic adenosine 3′, 5′-monophosphate production in isolated preovulatory rat follicles. Biol. Reprod. 39, 213–220 (1988)
Y.X. Liu, B.G. Kasson, K.D. Dahl, A.J.W. Hsueh, Vasoactive intestinal peptide stimulates plasminogen activator activity by cultured rat granulosa cells and cumulus-oocyte complexes. Peptides 8, 29–33 (1987)
G. Schmidt, J. Jörgensen, P. Kannisto, F. Liedberg, B. Ottesen, Ch. Owman, Vasoactive intestinal polypeptide in the PMSG-primed immature rat ovary and its effect on ovulation in the isolated rat ovary perfused in vitro. J. Reprod. Fertil. 90, 465–472 (1990)
J.A. Flaws, A. DeSanti, K.I. Tilly, R.O. Javid, K. Kugu, A.L. Johnson, A.N. Hirshfield, J.L. Tilly, Vasoactive intestinal peptide-mediated suppression of apoptosis in the ovary: potential mechanisms of action and evidence of a conserved antiatretogenic role through evolution. Endocrinology 136, 4351–4359 (1995)
C.M. Fredericks, L.E. Lundquist, R.S. Mathur, S.H. Ashton, S.C. Landgrebe, Effects of vasoactive intestinal peptide upon ovarian steroids, ovum transport, and fertility in the rabbit. Biol. Reprod. 28, 1052–1060 (1983)
A.L. Johnson, Z. Li, J.A. Gibney, S. Malamed, Vasoactive intestinal peptide-induced expression of cytochrome P450 cholesterol side-chain cleavage and 17α-hydroxylase enzyme activity in hen granulosa cells. Biol. Reprod. 51, 327–333 (1994)
J.B. Davoren, A.J.W. Hsueh, Vasoactive intestinal peptide: a novel stimulator of steroidogenesis by cultured rat granulosa cells. Biol. Reprod. 33, 37–52 (1985)
M.P. Kowalewski, M.T. Dyson, A. Boos, D.M. Stocco, Vasoactive intestinal peptide (VIP)-mediated expression and function of steroidogenic acute regulatory protein (StAR) in granulosa cells. Mol. Cell. Endocrinol. 328, 93–103 (2010)
F.W. George, S.R. Ojeda, Vasoactive intestinal peptide enhances aromatase activity in the neonatal rat ovary before development of primary follicles or responsiveness to follicle-stimulating hormone. Proc. Natl. Acad. Sci. USA 84, 5803–5807 (1987)
C. Parra, J.L. Fiedler, S.L. Luna, M. Greiner, V. Padmanabhan, H.E. Lara, Participation of vasoactive intestinal polypeptide in ovarian steroids production during the rat estrous cycle and in the development of estradiol valerate-induced polycystic ovary. Reproduction 133, 147–154 (2007)
A.I. Barco, A. Flores, R. Chavira, P. Damián-Matsumara, R. Domínguez, M.E. Cruz, Asymmetric effects of acute hemiovariectomy on steroid hormone secretion by the in situ ovary. Endocrine 21, 209–215 (2003)
M.E. Cruz, A. Flores, M.T. Palafox, G. Meléndez, J.O. Rodríguez, R. Chavira, R. Domínguez, The role of the muscarinic system in regulation estradiol secretion varies during the estrous cycle: the hemiovariectomized rat model. Reprod. Biol. Endocrinol. 4, 43 (2006)
A. Flores, G. Meléndez, M.T. Palafox, J.O. Rodríguez, A.I. Barco, R. Chavira, R. Domínguez, M.E. Cruz, The participation of the cholinergic system in regulating progesterone secretion through the ovarian-adrenal crosstalk varies along the estrous cycle. Endocrine 28, 145–151 (2005)
A. Flores, J.O. Rodríguez, M.T. Palafox, G. Meléndez, A.I. Barco, R. Chavira, M.E. Cruz, R. Domínguez, The acute asymmetric effects of hemiovariectomy on testosterone secretion vary along the estrous cycle. The participation of the cholinergic system. Reprod. Biol. Endocrinol. 4, 11 (2006)
A. Flores, J. Velasco, A.I. Gallegos, F.D. Mendoza, P.M. Everardo, M.E. Cruz, R. Domínguez, Acute effects of unilateral sectioning the superior ovarian nerve of rats with unilateral ovariectomy on ovarian hormones (progesterone, testosterone and estradiol) levels vary during the estrous cycle. Reprod. Biol. Endocrinol. 9, 34 (2011)
R. Domínguez, M.E. Cruz, C. Morán, Differential effects of ovarian local anaesthesia during pro-oestrus on ovulation by the right or left ovary in normal and hemi-ovariectomized adult rats. J. Reprod. Fertil. 113, 185–190 (1998)
C. Morán, A. Franco, J.L. Morán, A. Handal, L. Morales, R. Domínguez, Neural activity between ovaries and the prevertebral celiac-superior mesenteric ganglia varies during the estrous cycle of the rat. Endocrine 26, 147–152 (2005)
C. Morán, F. Zarate, J.L. Morán, A. Handal, R. Domínguez, Lateralization of the connections of the ovary to the celiac ganglia in juvenile rats. Reprod. Biol. Endocrinol. 7, 50 (2009)
M.J. Moran, M.E. Ayala, E. Gallegos, J. Romero, R. Chavira, P. Damián-Matsumura, R. Domínguez, Effects of systemic administration or intrabursal injection of serotonin on puberty, first ovulation and follicular development in rats. Reprod. Fertil. Dev. 25, 1105–1114 (2013)
I. Gerendai, I.E. Tóth, Z. Boldogkoi, B. Halász, Recent findings on the organization of central nervous system structures involved in the innervation of endocrine glands and other organs; observations obtained by the transneuronal viral double-labeling technique. Endocrine 36, 179–188 (2009)
I.E. Tóth, P. Banczerowski, Z. Boldogkoi, J.S. Tóth, A. Szabó, B. Halász, I. Gerendai, Cerebral neurons involved in the innervation of both the adrenal gland and the ovary: a double viral tracing study. Brain Res. Bull. 77, 306–311 (2008)
A. Flores, A.I. Gallegos, J. Velasco, F.D. Mendoza, C. Montiel, P.M. Everardo, M.E. Cruz, R. Domínguez, The acute effects of bilateral ovariectomy or adrenalectomy on progesterone, testosterone and estradiol serum levels depend on the surgical approach and the day of the estrous cycle when they are performed. Reprod. Biol. Endocrinol. 6, 48 (2008)
J. Kalász, E.P. Tóth, B. Bódi, M. Fagyas, A. Tóth, B.H. Pal, S.G. Vári, M. Balog, S. Blažetić, M. Heffer, Z. Papp, A. Borbély, Single acute stress-induced progesterone and ovariectomy alter cardiomyocyte contractile function in female rats. Croat. Med. J. 55, 239–249 (2014)
W.H. Trzeciak, C.E. Ahmed, E.R. Simpson, S.R. Ojeda, Vasoactive intestinal peptide induces the synthesis of the cholesterol side-chain cleavage enzyme complex in cultured rat ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 83, 7490–7494 (1986)
M.H. Garraza, L.I. Aguado, M.A. De Bortoli, In vitro effect of neuropeptides on ovary or celiac ganglion affects the release of progesterone from ovaries in the rat. Med. Sci. Monit. 10, 440–446 (2004)
J. Hu, Z. Zhang, W.J. Shen, S. Azhar, Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 7, 47 (2010)
F. Stahl, F. Götz, G. Dörner, The influence of fetal adrenals on the androgen levels during brain differentiation in human subjects and rats. Exp. Clin. Endocrinol. 98, 131–139 (1991)
S. Uchida, F. Kagitani, H. Hotta, T. Hanada, Y. Aikawa, Cutaneous mechanical stimulation regulates ovarian blood flow via activation of spinal and supraspinal reflex pathways in anesthetized rats. Jpn. J. Physiol. 55, 265–277 (2005)
L.I. Aguado, S.R. Ojeda, Ovarian adrenergic nerves play a role in maintaining preovulatory steroid secretion. Endocrinology 114, 1944–1946 (1984)
J.P. Advis, C.E. Ahmed, S.R. Ojeda, Direct hypothalamic control of vasoactive intestinal peptide (VIP) levels in the developing rat ovary. Brain Res. Bull. 22, 605–610 (1989)
J.K. Findlay, K. Britt, J.B. Kerr, L. O’Donnell, M.E. Jones, A.E. Drummond, E.R. Simpson, The road to ovulation: the role of oestrogens. Reprod. Fertil. Dev. 13, 543–547 (2001)
B.G. Kasson, R. Meidan, J.B. Davoren, A.J. Hsueh, Identification of subpopulations of rat granulosa cells: sedimentation properties and hormonal responsiveness. Endocrinology 117, 1027–1034 (1985)
S. Uchida, F. Kagitani, H. Hotta, Reflex modulation of ovarian estradiol secretion by noxious mechanical stimulation of a hindpaw in anesthetized rats. Auton. Neurosci. 171, 14–20 (2012)
S. Uchida, F. Kagitani, Autonomic nervous regulation of ovarian function by noxious somatic afferent stimulation. J. Physiol. Sci. (2014). doi:10.1007/s12576-014-0324-9
I.E. Tóth, O. Wiesel, Z. Boldogkoi, K. Bálint, Z. Tapaszti, I. Gerendai, Predominance of supraspinal innervation of the left ovary. Microsc. Res. Tech. 70, 710–718 (2007)
C.M. Klein, H.W. Burden, Anatomical localization of afferent and postganglionic sympathetic neurons innervating the rat ovary. Neurosci. Lett. 85, 217–222 (1988)
Acknowledgments
This work was supported by grant UNAM-DGAPA-PAPIIT IN211813. We want to thank for the support given to in the realization of this study to the “Posgrado en Ciencias Biológicas, UNAM” and CONACyT. This work is a requirement for obtaining the degree of Doctor of Biological Sciences. We also thank Biol. R. Chavira having participated in performing the RIA’s to measure the hormones levels. We also want to thank M Sc A. Domínguez-González for the revision of the manuscript in English.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosas, G., Ramírez, M.I., Linares, R. et al. Asymmetric steroidogenic response by the ovaries to the vasoactive intestinal peptide. Endocrine 48, 968–977 (2015). https://doi.org/10.1007/s12020-014-0449-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0449-x