Abstract
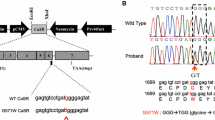
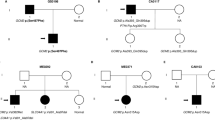
The calcium-sensing receptor (CaSR) is a G-protein-coupled receptor with a crucial role in calcium homeostasis. Mutations in the CaSR gene may lead to specific parathyroid disorders due to either gain-of-function (autosomal dominant hypercalciuric hypocalcemia; ADHH) or loss-of-function (familial hypocalciuric hypercalcemia; FHH). Our aim was to evaluate CaSR mutations as a cause of disease in selected patients. We identified and recruited patients with phenotypes suggestive of CaSR-related parathyroid disorders. DNA was extracted, and CaSR gene was sequenced. Live-ratiometric measurements of intracellular [Ca2+] and Western blot assays for evaluation of MAPK phosphorylation in response to changes in extracellular [Ca2+] were performed in transiently transfected HEK-293T cells to functionally characterize mutants. A total of 21 patients were evaluated, seven of them with idiopathic hypoparathyroidism (suspected ADHH) and 14 with hyperparathyroidism (suspected FHH). In the latter group two patients were found to harbor missense mutations: a novel heterozygous I32 V mutation in a female index case and a sporadic known R185Q mutation in a 1-year-old girl. In-vitro functional studies showed that I32 V is an inactivating mutation. In our study, most patients had normal CaSR sequencing. This suggests that phenotypic pitfalls may occur at time of patients’ selection for CaSR sequencing. In one patient with strong positive pre-test probability based on both familial history and appropriate phenotype, a novel I32 V mutation leading to FHH was identified and characterized. In cases of familial parathyroid disorders, CaSR sequencing should be performed, but if negative, one should consider involvement of alternative genes or mechanisms.




Similar content being viewed by others
References
N. Chattopadhyay, A. Mithal, E.M. Brown, The calcium-sensing receptor: a window into the physiology and pathophysiology of mineral ion metabolism. Endocr. Rev. 17(4), 289–307 (1996)
D. Riccardi, E.M. Brown, Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. Physiol. Renal. Physiol. 298(3), F485–F499 (2009)
A. Hofer, E. Brown, Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 4(7), 530–538 (2003)
C. Ye, K. Rogers, M. Bai, S.J. Quinn, E.M. Brown, P.M. Vassilev, Agonists of the Ca(2+)-sensing receptor (CaR) activate nonselective cation channels in HEK293 cells stably transfected with the human CaR. Biochem. Biophys. Res. Commun. 226(2), 572–579 (1996)
A.L. Magno, B.K. Ward, T. Ratajczak, The calcium-sensing receptor: a molecular perspective. Endocr. Rev. 32(1), 3–30 (2011)
E. Brown, Clinical lessons from the calcium-sensing receptor. Nat. Clin. Pract. Endocrinol. Metab. 3(2), 122–133 (2007)
M. Yamamoto, T. Akatsu, T. Nagase, E. Ogata, Comparison of hypocalcemic hypercalciuria between patients with idiopathic hypoparathyroidism and those with gain-of-function mutations in the calcium-sensing receptor: is it possible to differentiate the two disorders? J. Clin. Endocrinol. Metab. 85(12), 4583–4591 (2000)
I.R. Gunn, D. Gaffney, Clinical and laboratory features of calcium-sensing receptor disorders: a systematic review. Ann. Clin. Biochem. 41(Pt 6), 441–458 (2004)
O. Egbuna, E. Brown, Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract. Res. Clin. Rheumatol. 22(1), 129–148 (2008)
J. Varghese, T. Rich, C. Jimenez, Benign familial hypocalciuric hypercalcemia. Endocr. Pract. 17(Suppl 1), 13–17 (2011)
U. Quitterer, M. Hoffmann, M. Freichel, M.J. Lohse, Paradoxical block of parathormone secretion is mediated by increased activity of G alpha subunits. J. Biol. Chem. 276(9), 6763–6769 (2001)
S.H. Pearce, M. Bai, S.J. Quinn, O. Kifor, E.M. Brown, R.V. Thakker, Functional characterization of calcium-sensing receptor mutations expressed in human embryonic kidney cells. J. Clin. Investig. 98(8), 1860–1866 (1996)
I.A. Adzhubei, S. Schmidt, L. Peshkin, V.E. Ramensky, A. Gerasimova, P. Bork, A.S. Kondrashov, S.R. Sunyaev, A method and server for predicting damaging missense mutations. Nat. Methods 7(4), 248–249 (2010)
J.M. Schwarz, C. Rodelsperger, M. Schuelke, D. Seelow, Mutationtaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7(8), 575–576 (2010)
M. Bai, S. Trivedi, E.M. Brown, Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem. 273(36), 23605–23610 (1998)
M.R. Pollak, E.M. Brown, Y.H. Chou, S.C. Hebert, S.J. Marx, B. Steinmann, T. Levi, C.E. Seidman, J.G. Seidman, Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 75(7), 1297–1303 (1993)
H. Heath 3rd, S. Odelberg, C.E. Jackson, B.T. Teh, N. Hayward, C. Larsson, N.R. Buist, K.J. Krapcho, B.C. Hung, I.V. Capuano, J.E. Garrett, M.F. Leppert, Clustered inactivating mutations and benign polymorphisms of the calcium receptor gene in familial benign hypocalciuric hypercalcemia suggest receptor functional domains. J. Clin. Endocrinol. Metab. 81(4), 1312–1317 (1996)
B. Obermannova, K. Banghova, Z. Sumnik, H.M. Dvorakova, J. Betka, F. Fencl, S. Kolouskova, O. Cinek, J. Lebl, Unusually severe phenotype of neonatal primary hyperparathyroidism due to a heterozygous inactivating mutation in the CASR gene. Eur. J. Pediatr. 168(5), 569–573 (2009)
C.M. Reh, G.N. Hendy, D.E. Cole, D.D. Jeandron, Neonatal hyperparathyroidism with a heterozygous calcium-sensing receptor (CASR) R185Q mutation: clinical benefit from cinacalcet. J. Clin. Endocrinol. Metab. 96(4), E707–E712 (2011)
M. Bai, S.H. Pearce, O. Kifor, S. Trivedi, U.G. Stauffer, R.V. Thakker, E.M. Brown, B. Steinmann, In vivo and in vitro characterization of neonatal hyperparathyroidism resulting from a de novo, heterozygous mutation in the Ca2+-sensing receptor gene: normal maternal calcium homeostasis as a cause of secondary hyperparathyroidism in familial benign hypocalciuric hypercalcemia. J. Clin. Investig. 99(1), 88–96 (1997)
S.E. Christensen, P.H. Nissen, P. Vestergaard, L. Heickendorff, K. Brixen, L. Mosekilde, Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clin. Endocrinol. (Oxf) 69(5), 713–720 (2008)
Y.H. Chou, M.R. Pollak, M.L. Brandi, G. Toss, H. Arnqvist, A.B. Atkinson, S.E. Papapoulos, S. Marx, E.M. Brown, J.G. Seidman et al., Mutations in the human Ca(2+)-sensing-receptor gene that cause familial hypocalciuric hypercalcemia. Am. J. Hum. Genet. 56(5), 1075–1079 (1995)
V. Guarnieri, L. Canaff, F.H. Yun, A. Scillitani, C. Battista, L.A. Muscarella, B.Y. Wong, A. Notarangelo, L. D’Agruma, M. Sacco, D.E. Cole, G.N. Hendy, Calcium-sensing receptor (CASR) mutations in hypercalcemic states: studies from a single endocrine clinic over three years. J. Clin. Endocrinol. Metab. 95(4), 1819–1829 (2010)
S.H. Pearce, D. Trump, C. Wooding, G.M. Besser, S.L. Chew, D.B. Grant, D.A. Heath, I.A. Hughes, C.R. Paterson, M.P. Whyte et al., Calcium-sensing receptor mutations in familial benign hypercalcemia and neonatal hyperparathyroidism. J. Clin. Investig. 96(6), 2683–2692 (1995)
K. Frank-Raue, G. Leidig-Bruckner, C. Haag, E. Schulze, A. Lorenz, H. Schmitz-Winnenthal, F. Raue, Inactivating calcium-sensing receptor mutations in patients with primary hyperparathyroidism. Clin. Endocrinol. (Oxf) 75(1), 50–55 (2011)
F. Raue, J. Pichl, H.G. Dorr, D. Schnabel, P. Heidemann, G. Hammersen, C. Jaursch-Hancke, R. Santen, C. Schofl, M. Wabitsch, C. Haag, E. Schulze, K. Frank-Raue, Activating mutations in the calcium-sensing receptor: genetic and clinical spectrum in 25 patients with autosomal dominant hypocalcaemia-a German survey. Clin. Endocrinol. (Oxf) 75(6), 760–765 (2011)
R. Sarin, N. Tomar, D. Ray, N. Gupta, Y.D. Sharma, R. Goswami, Absence of pathogenic calcium sensing receptor mutations in sporadic idiopathic hypoparathyroidism. Clin. Endocrinol. (Oxf) 65(3), 359–363 (2006)
A. Lienhardt, M. Bai, J. Lagarde, M. Rigaud, Z. Zhang, Y. Jiang, M. Kottler, E. Brown, M. Garabédian, Activating mutations of the calcium-sensing receptor: management of hypocalcemia. J. Clin. Endocrinol. Metab. 86(11), 5313–5323 (2001)
R. Eastell, A. Arnold, M.L. Brandi, E.M. Brown, P. D’Amour, D.A. Hanley, D.S. Rao, M.R. Rubin, D. Goltzman, S.J. Silverberg, S.J. Marx, M. Peacock, L. Mosekilde, R. Bouillon, E.M. Lewiecki, Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J. Clin. Endocrinol. Metab. 94(2), 340–350 (2009)
K. Sato, Y. Hasegawa, J. Nakae, K. Nanao, I. Takahashi, T. Tajima, N. Shinohara, K. Fujieda, Hydrochlorothiazide effectively reduces urinary calcium excretion in two Japanese patients with gain-of-function mutations of the calcium-sensing receptor gene. J. Clin. Endocrinol. Metab. 87(7), 3068–3073 (2002)
A. Szalat, H. Mazeh, H.R. Freund, Lithium-associated hyperparathyroidism: report of four cases and review of the literature. Eur. J. Endocrinol. 160(2), 317–323 (2009)
M.A. Nesbit, F.M. Hannan, S.A. Howles, V.N. Babinsky, R.A. Head, T. Cranston, N. Rust, M.R. Hobbs, H. Heath 3rd, R.V. Thakker, Mutations affecting G-protein subunit alpha11 in hypercalcemia and hypocalcemia. N. Engl. J. Med. 368(26), 2476–2486 (2013)
M.A. Nesbit, F.M. Hannan, S.A. Howles, A.A. Reed, T. Cranston, C.E. Thakker, L. Gregory, A.J. Rimmer, N. Rust, U. Graham, P.J. Morrison, S.J. Hunter, M.P. Whyte, G. McVean, D. Buck, R.V. Thakker, Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat. Genet. 45(1), 93–97 (2013)
J.P. Bilezikian, A. Khan, J.T. Potts Jr, M.L. Brandi, B.L. Clarke, D. Shoback, H. Juppner, P. D’Amour, J. Fox, L. Rejnmark, L. Mosekilde, M.R. Rubin, D. Dempster, R. Gafni, M.T. Collins, J. Sliney, J. Sanders, Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J. Bone Miner. Res. 26(10), 2317–2337 (2011)
R. Goswami, E. Brown, N. Kochupillai, N. Gupta, R. Rani, O. Kifor, N. Chattopadhyay, Prevalence of calcium sensing receptor autoantibodies in patients with sporadic idiopathic hypoparathyroidism. Eur. J. Endocrinol. 150(1), 9–18 (2004)
A. Mayer, C. Ploix, J. Orgiazzi, A. Desbos, A. Moreira, H. Vidal, J.C. Monier, J. Bienvenu, N. Fabien, Calcium-sensing receptor autoantibodies are relevant markers of acquired hypoparathyroidism. J. Clin. Endocrinol. Metab. 89(9), 4484–4488 (2004)
N. Gavalas, E. Kemp, K. Krohn, E. Brown, P. Watson, A. Weetman, The calcium-sensing receptor is a target of autoantibodies in patients with autoimmune polyendocrine syndrome type 1. J. Clin. Endocrinol. Metab. 92(6), 2107–2114 (2007)
O. Kifor, F.D. Moore Jr, M. Delaney, J. Garber, G.N. Hendy, R. Butters, P. Gao, T.L. Cantor, I. Kifor, E.M. Brown, J. Wysolmerski, A syndrome of hypocalciuric hypercalcemia caused by autoantibodies directed at the calcium-sensing receptor. J. Clin. Endocrinol. Metab. 88(1), 60–72 (2003)
J. Pallais, O. Kifor, Y. Chen, D. Slovik, E. Brown, Acquired hypocalciuric hypercalcemia due to autoantibodies against the calcium-sensing receptor. N. Engl. J. Med. 351(4), 362–369 (2004)
J.C. Pallais, E.H. Kemp, C. Bergwitz, L. Kantham, D.M. Slovik, A.P. Weetman, E.M. Brown, Autoimmune hypocalciuric hypercalcemia unresponsive to glucocorticoid therapy in a patient with blocking autoantibodies against the calcium-sensing receptor. J. Clin. Endocrinol. Metab. 96(3), 672–680 (2010)
M. Alimohammadi, P. Björklund, A. Hallgren, N. Pöntynen, G. Szinnai, N. Shikama, M.P. Keller, O. Ekwall, S.A. Kinkel, E.S. Husebye, J. Gustafsson, F. Rorsman, L. Peltonen, C. Betterle, J. Perheentupa, G. Akerström, G. Westin, H.S. Scott, G.A. Holländer, O. Kämpe, Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N. Eng. J. Med. 358(10), 1018–1028 (2008)
N. Tomar, E. Kaushal, M. Das, N. Gupta, C. Betterle, R. Goswami, Prevalence and significance of NALP5 autoantibodies in patients with idiopathic hypoparathyroidism. J. Clin. Endocrinol. Metab. 97(4), 1219–1226 (2012)
Acknowledgments
The authors would like to thank Prof. Tally Naveh-Many and Prof. Justin Silver for technical assistance, as well as Pr. Benjamin Glaser for support of the study and critical review of the manuscript. The authors also thank Dr. Victoria Terletzky, Dr. Anat Tsur, Dr. Larissa Shustin, Dr. Merav Frankel, Prof. Yair Liel, Dr. Rivka Backenroth-Maayan, Dr Leanna Shkolnik-Tripto, Prof. Iris Vered, and Prof. Yossef Weisman, for cooperation in the collection of clinical and laboratory data. Thanks to Shirley smith for the help in writing the manuscript. This work was supported by the Research Foundation of the Chief Scientist at the Israel Ministry of Health.
Conflict of interest
The authors disclose no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szalat, A., Shahar, M., Shpitzen, S. et al. Calcium-sensing receptor sequencing in 21 patients with idiopathic or familial parathyroid disorder: pitfalls and characterization of a novel I32 V loss-of-function mutation. Endocrine 48, 444–453 (2015). https://doi.org/10.1007/s12020-014-0370-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0370-3