Abstract
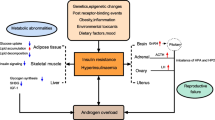
Polycystic ovary syndrome (PCOS) is an endocrine disease of women in reproductive age. It is characterized by anovulation and hyperandrogenism. Most often patients with PCOS have metabolic abnormalities such as dyslipidemia, insulin resistance, and glucose intolerance. It is not surprising that obesity is high prevalent in PCOS. Over 60 % of PCOS women are obese or overweight. Modulation of appetite and energy intake is essential to maintain energy balance and body weight. The gastrointestinal tract, where nutrients are digested and absorbed, plays a central role in energy homeostasis. The signals from the gastrointestinal tract arise from the stomach (ghrelin release), proximal small intestine (CCK release), and distal small intestine (GLP-1 and PYY) in response to food. These hormones are recognized as “appetite regulatory hormones.” Weight loss is the key in the treatments of obese/overweight patients with PCOS. However, current non-pharmacologic management of body weight is hard to achieve. This review highlighted the gastrointestinal hormones, and discussed the potential strategies aimed at modifying hormones for treatment in PCOS.


Similar content being viewed by others
References
E.S. Knochenhauer, T.J. Key, M. Kahsar-Miller, W. Waggoner, L.R. Boots, R. Azziz, Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J. Clin. Endocrinol. Metab. 83, 3078–3082 (1998)
E. Diamanti-Kandarakis, C.R. Kouli, A.T. Bergiele, F.A. Filandra, T.C. Tsianateli, G.G. Spina, E.D. Zapanti, M.I. Bartzis, A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 84, 4006–4011 (1999)
W.A. March, V.M. Moore, K.J. Willson, D.I. Phillips, R.J. Norman, M.J. Davies, The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 25, 544–551 (2010)
H. Jia, L. Yu, X. Guo, W. Gao, Z. Jiang, Associations of adiponectin gene polymorphisms with polycystic ovary syndrome: a meta-analysis. Endocrine 42, 299–306 (2012)
M. Brower, K. Brennan, M. Pall, R. Azziz, The severity of menstrual dysfunction as a predictor of insulin resistance in PCOS. J. Clin. Endocrinol. Metab. 98, E1967–E1971 (2013)
S.F. Witchel, S.E. Recabarren, F. Gonzalez, E. Diamanti-Kandarakis, K.I. Cheang, A.J. Duleba, R.S. Legro, R. Homburg, R. Pasquali, R.A. Lobo, C.C. Zouboulis, F. Kelestimur, F. Fruzzetti, W. Futterweit, R.J. Norman, D.H. Abbott, Emerging concepts about prenatal genesis, aberrant metabolism and treatment paradigms in polycystic ovary syndrome. Endocrine 42, 526–534 (2012)
S.S. Lim, R.J. Norman, M.J. Davies, L.J. Moran, The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes. Rev. 14, 95–109 (2013)
N. Kanaya, S. Vonderfecht, S. Chen, Androgen (dihydrotestosterone)-mediated regulation of food intake and obesity in female mice. J.Steroid Biochem. Mol. Biol. 138, 100–106 (2013)
A.L. Hirschberg, S. Naessen, M. Stridsberg, B. Bystrom, J. Holtet, Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol. Endocrinol. 19, 79–87 (2004)
D. Mahoney, Lifestyle modification intervention among infertile overweight and obese women with polycystic ovary syndrome. J. Am. Assoc. Nurse Pract. (2013). doi:10.1002/2327-6924.12073
B.A. Gower, P.C. Chandler-Laney, F. Ovalle, L.L. Goree, R. Azziz, R.A. Desmond, W.M. Granger, A.M. Goss, G.W. Bates, Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin. Endocrinol. 79, 550–557 (2013)
P.J. Havel, Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp. Biol. Med. (Maywood) 226, 963–977 (2001)
R. Deniz, B. Gurates, S. Aydin, H. Celik, I. Sahin, Y. Baykus, Z. Catak, A. Aksoy, C. Citil, S. Gungor, Nesfatin-1 and other hormone alterations in polycystic ovary syndrome. Endocrine 42, 694–699 (2012)
S. Aydin, Multi-functional peptide hormone NUCB2/nesfatin-1. Endocrine 44, 312–325 (2013)
M. Tschop, D.L. Smiley, M.L. Heiman, Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000)
G. Gomez, E.W. Englander, G.H. Greeley Jr, Nutrient inhibition of ghrelin secretion in the fasted rat. Regul. Pept. 117, 33–36 (2004)
J. Overduin, R.S. Frayo, H.J. Grill, J.M. Kaplan, D.E. Cummings, Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146, 845–850 (2005)
B.A. Parker, S. Doran, J. Wishart, M. Horowitz, I.M. Chapman, Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin. Endocrinol. 62, 539–546 (2005)
W.A. Blom, A. Stafleu, C. de Graaf, F.J. Kok, G. Schaafsma, H.F. Hendriks, Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am. J. Clin. Nutr. 81, 367–375 (2005)
Y. Greenman, N. Golani, S. Gilad, M. Yaron, R. Limor, N. Stern, Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin. Endocrinol. 60, 382–388 (2004)
A.M. Wren, L.J. Seal, M.A. Cohen, A.E. Brynes, G.S. Frost, K.G. Murphy, W.S. Dhillo, M.A. Ghatei, S.R. Bloom, Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 86, 5992 (2001)
D.E. Cummings, K. Clement, J.Q. Purnell, C. Vaisse, K.E. Foster, R.S. Frayo, M.W. Schwartz, A. Basdevant, D.S. Weigle, Elevated plasma ghrelin levels in Prader Willi syndrome. Nat. Med. 8, 643–644 (2002)
D.E. Cummings, D.S. Weigle, R.S. Frayo, P.A. Breen, M.K. Ma, E.P. Dellinger, J.Q. Purnell, Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 346, 1623–1630 (2002)
P.J. Currie, A. Mirza, R. Fuld, D. Park, J.R. Vasselli, Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R353–R358 (2005)
M. Nakazato, N. Murakami, Y. Date, M. Kojima, H. Matsuo, K. Kangawa, S. Matsukura, A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001)
M.A. Cowley, J.L. Smart, M. Rubinstein, M.G. Cerdan, S. Diano, T.L. Horvath, R.D. Cone, M.J. Low, Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001)
D.E. Cummings, K.E. Foster, Ghrelin-leptin tango in body-weight regulation. Gastroenterology 124, 1532–1535 (2003)
A. Moulin, L. Brunel, D. Boeglin, L. Demange, J. Ryan, C. M’Kadmi, S. Denoyelle, J. Martinez, J.A. Fehrentz, The 1,2,4-triazole as a scaffold for the design of ghrelin receptor ligands: development of JMV 2959, a potent antagonist. Amino Acids 44, 301–314 (2013)
G. Arusoglu, G. Koksal, N. Cinar, S. Tapan, D.Y. Aksoy, B.O. Yildiz, Basal and meal-stimulated ghrelin, PYY, CCK levels and satiety in lean women with polycystic ovary syndrome: effect of low-dose oral contraceptive. J. Clin. Endocrinol. Metab. 98, 4475–4482 (2013)
Japur, CC, Diez-Garcia, RW, de Oliveira Penaforte, FR, de Sa, MF, Imbalance between postprandial ghrelin and insulin responses to an ad libitum meal in obese women with polycystic ovary syndrome. Reprod. Sci. (2014) [Epub ahead of print]
I.T. Ozgen, M. Aydin, A. Guven, Y. Aliyazicioglu, Characteristics of polycystic ovarian syndrome and relationship with ghrelin in adolescents. J. Pediatr. Adolesc. Gynecol. 23, 285–289 (2010)
T.M. Barber, F.F. Casanueva, F. Karpe, M. Lage, S. Franks, M.I. McCarthy, J.A. Wass, Ghrelin levels are suppressed and show a blunted response to oral glucose in women with polycystic ovary syndrome. Eur. J. Endocrinol 158, 511–516 (2008)
M. Mitkov, B. Pehlivanov, Orbetzova, M:Serum ghrelin level in women with polycystic ovary syndrome and its relationship with endocrine and metabolic parameters. Gynecol. Endocrinol. 24, 625–630 (2008)
A. Bideci, M.O. Camurdan, E. Yesilkaya, F. Demirel, P. Cinaz, Serum ghrelin, leptin and resistin levels in adolescent girls with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 34, 578–584 (2008)
M.J. Theodorakis, O. Carlson, S. Michopoulos, M.E. Doyle, M. Juhaszova, K. Petraki, J.M. Egan, Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am. J. Physiol. Endocrinol. Metab. 290, E550–E559 (2006)
C.F. Deacon, What do we know about the secretion and degradation of incretin hormones? Regul. Pept. 128, 117–124 (2005)
R. Mentlein, B. Gallwitz, W.E. Schmidt, Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 214, 829–835 (1993)
R. Mentlein, Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul. Pept. 85, 9–24 (1999)
L. Hansen, C.F. Deacon, C. Orskov, J.J. Holst, Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140, 5356–5363 (1999)
L.A. Scrocchi, T.J. Brown, N. MaClusky, P.L. Brubaker, A.B. Auerbach, A.L. Joyner, D.J. Drucker, Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 2, 1254–1258 (1996)
R. Perfetti, J. Zhou, M.E. Doyle, J.M. Egan, Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141, 4600–4605 (2000)
J. Schirra, M. Nicolaus, R. Roggel, M. Katschinski, M. Storr, H.J. Woerle, B. Goke, Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55, 243–251 (2006)
J.J. Meier, B. Gallwitz, S. Salmen, O. Goetze, J.J. Holst, W.E. Schmidt, M.A. Nauck, Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 88, 2719–2725 (2003)
M.A. Nauck, U. Niedereichholz, R. Ettler, J.J. Holst, C. Orskov, R. Ritzel, W.H. Schmiegel, Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 273, E981–E988 (1997)
J.J. Hwa, L. Ghibaudi, P. Williams, M.B. Witten, R. Tedesco, C.D. Strader, Differential effects of intracerebroventricular glucagon-like peptide-1 on feeding and energy expenditure regulation. Peptides 19, 869–875 (1998)
F. Rodriquez de Fonseca, M. Navarro, E. Alvarez, I. Roncero, J.A. Chowen, O. Maestre, R. Gomez, R.M. Munoz, J. Eng, E. Blazquez, Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 49, 709–717 (2000)
J.J. Meier, M.A. Nauck, Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab. Res. Rev. 21, 91–117 (2005)
E. Naslund, M. Gutniak, S. Skogar, S. Rossner, P.M. Hellstrom, Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am. J. Clin. Nutr. 68, 525–530 (1998)
M.A. Nauck, J.J. Meier, Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regul. Pept. 128, 135–148 (2005)
M. Nauck, F. Stockmann, R. Ebert, W. Creutzfeldt, Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29, 46–52 (1986)
J. Ma, A.N. Pilichiewicz, C. Feinle-Bisset, J.M. Wishart, K.L. Jones, M. Horowitz, C.K. Rayner, Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet. Med. 29, 604–608 (2012)
L.R. Ranganath, J.M. Beety, L.M. Morgan, J.W. Wright, R. Howland, V. Marks, Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38, 916–919 (1996)
S. Madsbad, The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabet. Obes. Metab. 16, 9–21 (2014)
F.K. Knop, K. Aaboe, T. Vilsboll, A. Volund, J.J. Holst, T. Krarup, S. Madsbad, Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabet. Obes. Metab. 14, 500–510 (2012)
T. Vilsboll, T. Krarup, J. Sonne, S. Madsbad, A. Volund, A.G. Juul, J.J. Holst, Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 88, 2706–2713 (2003)
C. Verdich, S. Toubro, B. Buemann, L. Madsen, J. Juul, J. Holst, A. Astrup, The role of postprandial releases of insulin and incretin hormones in meal-induced satiety–effect of obesity and weight reduction. Int. J. Obes. Relat. Metab. Disord. 25, 1206–1214 (2001)
Y. Anini, P.L. Brubaker, Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52, 252–259 (2003)
J.G. Barrera, D.A. Sandoval, D.A. D’Alessio, R.J. Seeley, GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat. Rev. Endocrinol. 7, 507–516 (2011)
R. Gama, F. Norris, J. Wright, L. Morgan, S. Hampton, S. Watkins, V. Marks, The entero-insular axis in polycystic ovarian syndrome. Ann. Clin. Biochem. 33(Pt 3), 190–195 (1996)
C. Pontikis, M.P. Yavropoulou, K.A. Toulis, K. Kotsa, K. Kazakos, A. Papazisi, A. Gotzamani-Psarakou, J.G. Yovos, The incretin effect and secretion in obese and lean women with polycystic ovary syndrome: a pilot study. J. Women’s Health 20, 971–976 (2011)
J. Vrbikova, M. Hill, B. Bendlova, T. Grimmichova, K. Dvorakova, K. Vondra, G. Pacini, Incretin levels in polycystic ovary syndrome. Eur. J. Endocrinol. 159, 121–127 (2008)
J.F. Rehfeld, G. Sun, T. Christensen, J.G. Hillingso, The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J. Clin. Endocrinol. Metab. 86, 251–258 (2001)
R.A. Liddle, I.D. Goldfine, M.S. Rosen, R.A. Taplitz, J.A. Williams, Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Invest. 75, 1144–1152 (1985)
T.H. Moran, K.P. Kinzig, Gastrointestinal satiety signals II. Cholecystokinin. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G183–G188 (2004)
R.A. Liddle, Regulation of cholecystokinin secretion in humans. J. Gastroenterol. 35, 181–187 (2000)
C. Feinle, M. D’Amato, N.W. Read, Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology 110, 1379–1385 (1996)
W. Schwizer, J. Borovicka, P. Kunz, R. Fraser, C. Kreiss, M. D’Amato, G. Crelier, P. Boesiger, M. Fried, Role of cholecystokinin in the regulation of liquid gastric emptying and gastric motility in humans: studies with the CCK antagonist loxiglumide. Gut 41, 500–504 (1997)
C.K. Rayner, H.S. Park, S.M. Doran, I.M. Chapman, M. Horowitz, Effects of cholecystokinin on appetite and pyloric motility during physiological hyperglycemia. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G98–G104 (2000)
J. Glatzle, Y. Wang, D.W. Adelson, T.J. Kalogeris, T.T. Zittel, P. Tso, J.Y. Wei, H.E. Raybould, Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J. Physiol. 550, 657–664 (2003)
J.G. Gutierrez, W.Y. Chey, V.P. Dinoso, Actions of cholecystokinin and secretin on the motor activity of the small intestine in man. Gastroenterology 67, 35–41 (1974)
J. Gibbs, R.C. Young, G.P. Smith, Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature 245, 323–325 (1973)
T.H. Moran, P.J. Ameglio, G.J. Schwartz, P.R. McHugh, Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am. J. Physiol. 262, R46–R50 (1992)
T.H. Moran, S. Bi, Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1211–1218 (2006)
K. Meereis-Schwanke, H. Klonowski-Stumpe, L. Herberg, C. Niederau, Long-term effects of CCK-agonist and -antagonist on food intake and body weight in Zucker lean and obese rats. Peptides 19, 291–299 (1998)
L. Wang, M.D. Barachina, V. Martinez, J.Y. Wei, Y. Tache, Synergistic interaction between CCK and leptin to regulate food intake. Regul. Pept. 92, 79–85 (2000)
I.M. Brennan, T.J. Little, K.L. Feltrin, A.J. Smout, J.M. Wishart, M. Horowitz, C. Feinle-Bisset, Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 295, E1487–E1494 (2008)
C. Beglinger, L. Degen, D. Matzinger, M. D’Amato, J. Drewe, Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1149–R1154 (2001)
F.P. O’Harte, M.H. Mooney, C.M. Kelly, P.R. Flatt, Glycated cholecystokinin-8 has an enhanced satiating activity and is protected against enzymatic degradation. Diabetes 47, 1619–1624 (1998)
I.A. Montgomery, N. Irwin, P.R. Flatt, Beneficial effects of (pGlu-Gln)-CCK-8 on energy intake and metabolism in high fat fed mice are associated with alterations of hypothalamic gene expression. Hormon. Metab. Res. 45(6), 471–473 (2013)
N. Irwin, I.A. Montgomery, R.C. Moffett, P.R. Flatt, Chemical cholecystokinin receptor activation protects against obesity-diabetes in high fat fed mice and has sustainable beneficial effects in genetic ob/ob mice. Biochem. Pharmacol. 85, 81–91 (2013)
N. Irwin, P.R. Flatt, Enteroendocrine hormone mimetics for the treatment of obesity and diabetes. Curr. Opin. Pharmacol. 13, 989–995 (2013)
N. Irwin, P. Frizelle, F.P. O’Harte, P.R. Flatt, (pGlu-Gln)-CCK-8[mPEG]: a novel, long-acting, mini-PEGylated cholecystokinin (CCK) agonist that improves metabolic status in dietary-induced diabetes. Biochim. et Biophys. Acta 1830, 4009–4016 (2013)
B. Bidzinska-Speichert, A. Lenarcik, U. Tworowska-Bardzinska, R. Slezak, G. Bednarek-Tupikowska, A. Milewicz, Pro12Ala PPAR gamma2 gene polymorphism in PCOS women: the role of compounds regulating satiety. Gynecol. Endocrinol. 28, 195–198 (2012)
J. Wen, S.F. Phillips, M.G. Sarr, L.J. Kost, Holst, JJ:PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am. J. Physiol. 269, G945–G952 (1995)
R.P. Vincent, C.W. le Roux, The satiety hormone peptide YY as a regulator of appetite. J. Clin. Pathol. 61, 548–552 (2008)
M.S. Huda, J.P. Wilding, J.H. Pinkney, Gut peptides and the regulation of appetite. Obes. Rev. 7, 163–182 (2006)
L. Degen, S. Oesch, M. Casanova, S. Graf, S. Ketterer, J. Drewe, C. Beglinger, Effect of peptide YY3-36 on food intake in humans. Gastroenterology 129, 1430–1436 (2005)
H.C. Lin, W.Y. Chey, X. Zhao, Release of distal gut peptide YY (PYY) by fat in proximal gut depends on CCK. Peptides 21, 1561–1563 (2000)
G.A. Eberlein, V.E. Eysselein, M. Schaeffer, P. Layer, D. Grandt, H. Goebell, W. Niebel, M. Davis, T.D. Lee, J.E. Shively et al., A new molecular form of PYY: structural characterization of human PYY(3–36) and PYY(1–36). Peptides 10, 797–803 (1989)
R.L. Conter, J.J. Roslyn, I.L. Taylor, Effects of peptide YY on gallbladder motility. Am. J. Physiol. 252, G736–G741 (1987)
N. Vrang, A.N. Madsen, M. Tang-Christensen, G. Hansen, P.J. Larsen, PYY(3–36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R367–R375 (2006)
T.H. Moran, U. Smedh, K.P. Kinzig, K.A. Scott, S. Knipp, E.E. Ladenheim, Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R384–R388 (2005)
N.M. Neary, C.J. Small, M.R. Druce, A.J. Park, S.M. Ellis, N.M. Semjonous, C.L. Dakin, K. Filipsson, F. Wang, A.S. Kent, G.S. Frost, M.A. Ghatei, S.R. Bloom, Peptide YY3-36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology 146, 5120–5127 (2005)
R.L. Batterham, M.A. Cowley, C.J. Small, H. Herzog, M.A. Cohen, C.L. Dakin, A.M. Wren, A.E. Brynes, M.J. Low, M.A. Ghatei, R.D. Cone, S.R. Bloom, Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418, 650–654 (2002)
R.L. Batterham, M.A. Cohen, S.M. Ellis, C.W. Le Roux, D.J. Withers, G.S. Frost, M.A. Ghatei, S.R. Bloom, Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 349, 941–948 (2003)
C.R. Abbott, C.J. Small, A.R. Kennedy, N.M. Neary, A. Sajedi, M.A. Ghatei, S.R. Bloom, Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res. 1043, 139–144 (2005)
C.W. le Roux, R.L. Batterham, S.J. Aylwin, M. Patterson, C.M. Borg, K.J. Wynne, A. Kent, R.P. Vincent, J. Gardiner, M.A. Ghatei, S.R. Bloom, Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147, 3–8 (2006)
C.L. Roth, P.J. Enriori, K. Harz, J. Woelfle, M.A. Cowley, T. Reinehr, Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J. Clin. Endocrinol. Metab. 90, 6386–6391 (2005)
E. Naslund, P. Gryback, P.M. Hellstrom, H. Jacobsson, J.J. Holst, E. Theodorsson, L. Backman, Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int. J. Obes. Relat. Metab. Disord. 21, 387–392 (1997)
S.L. Pedersen, P.G. Sasikumar, S. Chelur, B. Holst, A. Artmann, K.J. Jensen, N. Vrang, Peptide hormone isoforms: N-terminally branched PYY3–36 isoforms give improved lipid and fat-cell metabolism in diet-induced obese mice. J. Pept. Sci. 16, 664–673 (2010)
M.L. Addison, J.S. Minnion, J.C. Shillito, K. Suzuki, T.M. Tan, B.C. Field, N. Germain-Zito, C. Becker-Pauly, M.A. Ghatei, S.R. Bloom, K.G. Murphy, A role for metalloendopeptidases in the breakdown of the gut hormone, PYY 3–36. Endocrinology 152, 4630–4640 (2011)
T. Tsilchorozidou, R.L. Batterham, G.S. Conway, Metformin increases fasting plasma peptide tyrosine tyrosine (PYY) in women with polycystic ovarian syndrome (PCOS). Clin. Endocrinol. 69, 936–942 (2008)
K. Zwirska-Korczala, K. Sodowski, S.J. Konturek, D. Kuka, M. Kukla, T. Brzozowski, W. Cnota, E. Wozniak-Grygiel, J. Jaworek, R. Buldak, B. Rybus-Kalinowska, M. Fryczowski, Postprandial response of ghrelin and PYY and indices of low-grade chronic inflammation in lean young women with polycystic ovary syndrome. J. Physiol. Pharmacol. 59(Suppl 2), 161–178 (2008)
H. Lee, J.Y. Oh, Y.A. Sung, H. Chung, Is insulin resistance an intrinsic defect in asian polycystic ovary syndrome? Yonsei Med. J. 54, 609–614 (2013)
D. Panidis, K. Tziomalos, E. Papadakis, C. Vosnakis, P. Chatzis, I. Katsikis, Lifestyle intervention and anti-obesity therapies in the polycystic ovary syndrome: impact on metabolism and fertility. Endocrine 44, 583–590 (2013)
R.L. Thomson, J.D. Buckley, M. Noakes, P.M. Clifton, R.J. Norman, G.D. Brinkworth, The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 3373–3380 (2008)
K.A. Marsh, K.S. Steinbeck, F.S. Atkinson, P. Petocz, J.C. Brand-Miller, Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am. Erican J. Clin. Nutr. 92, 83–92 (2010)
F. Esfahanian, M.M. Zamani, R. Heshmat, F. Moini nia, Effect of metformin compared with hypocaloric diet on serum C-reactive protein level and insulin resistance in obese and overweight women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 39, 806–813 (2013)
S.E. Kasim-Karakas, W.M. Cunningham, A. Tsodikov, Relation of nutrients and hormones in polycystic ovary syndrome. Am. J. Clin. Nutr. 85, 688–694 (2007)
A. Karamanlis, R. Chaikomin, S. Doran, M. Bellon, F.D. Bartholomeusz, J.M. Wishart, K.L. Jones, M. Horowitz, C.K. Rayner, Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am. J. Clin. Nutr. 86, 1364–1368 (2007)
N. Phelan, A. O’Connor, T. Kyaw Tun, N. Correia, G. Boran, H.M. Roche, J. Gibney, Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 93, 652–662 (2011)
Y.H. Zheng, X.H. Wang, M.H. Lai, H. Yao, H. Liu, H.X. Ma, Effectiveness of abdominal acupuncture for patients with obesity-type polycystic ovary syndrome: a randomized controlled trial. J. Altern. Complement. Med. 19, 740–745 (2013)
H. Zhang, Y. Peng, Z. Liu, S. Li, Z. Lv, L. Tian, J. Zhu, X. Zhao, M. Chen, Effects of acupuncture therapy on abdominal fat and hepatic fat content in obese children: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. J. Altern. Complement. Med. 17, 413–420 (2011)
B. Xu, J.H. Yuan, Z.C. Liu, M. Chen, X.J. Wang, Effect of acupuncture on plasma peptide YY in the patient of simple obesity. Zhongguo zhen jiu (Chin. Acupunct. Moxib.) 25, 837–840 (2005)
F. Gucel, B. Bahar, C. Demirtas, S. Mit, C. Cevik, Influence of acupuncture on leptin, ghrelin, insulin and cholecystokinin in obese women: a randomised, sham-controlled preliminary trial. Acupunct. Med. 30, 203–207 (2012)
H. Abdi, B. Zhao, M. Darbandi, M. Ghayour-Mobarhan, S. Tavallaie, A.A. Rahsepar, S.M. Parizadeh, M. Safariyan, M. Nemati, M. Mohammadi, P. Abbasi-Parizad, S. Darbandi, S. Akhlaghi, G.A. Ferns, The effects of body acupuncture on obesity: anthropometric parameters, lipid profile, and inflammatory and immunologic markers. Sci.World. J. 2012, 603539 (2012)
S.H. Cho, J.S. Lee, L. Thabane, J. Lee, Acupuncture for obesity: a systematic review and meta-analysis. Int. J. Obes. 33, 183–196 (2009)
K.M. Hoeger, L. Kochman, N. Wixom, K. Craig, R.K. Miller, D.S. Guzick, A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil. Steril. 82, 421–429 (2004)
J. Xiao, S. Chen, C. Zhang, S. Chang, The effectiveness of metformin ovulation induction treatment in patients with PCOS: a systematic review and meta-analysis. Gynecol. Endocrinol. 28, 956–960 (2012)
M. Shaker, Z.I. Mashhadani, A.A. Mehdi, Effect of Treatment with Metformin on Omentin-1, Ghrelin and other Biochemical, Clinical Features in PCOS Patients. Oman Med. J. 25, 289–293 (2010)
P.F. Svendsen, L. Nilas, S. Madsbad, J.J. Holst, Incretin hormone secretion in women with polycystic ovary syndrome: roles of obesity, insulin sensitivity, and treatment with metformin. Metabolism 58, 586–593 (2009)
W. Wei, H. Zhao, A. Wang, M. Sui, K. Liang, H. Deng, Y. Ma, Y. Zhang, H. Zhang, Y. Guan, A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur. J. Endocrinol. 166, 99–105 (2012)
Y. An, Z. Sun, Y. Zhang, B. Liu, Y. Guan, M. Lu, The use of berberine for women with polycystic ovary syndrome undergoing IVF treatment. Clin. Endocrinol. 80, 425–431 (2014)
X. Zhang, Y. Zhao, M. Zhang, X. Pang, J. Xu, C. Kang, M. Li, C. Zhang, Z. Zhang, Y. Zhang, X. Li, G. Ning, L. Zhao, Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PloS One 7, e42529 (2012)
W. Xie, D. Gu, J. Li, K. Cui, Y. Zhang, Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6 J mice. PloS One 6, e24520 (2011)
T. Vilsboll, M. Christensen, A.E. Junker, F.K. Knop, L.L. Gluud, Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. Br. Med. J. 344, d7771 (2012)
M. Monami, I. Dicembrini, N. Marchionni, C.M. Rotella, E. Mannucci, Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp. Diabetes Res. 2012, 672658 (2012)
A.S. Kelly, K.D. Rudser, B.M. Nathan, C.K. Fox, A.M. Metzig, B.J. Coombes, A.K. Fitch, E.M. Bomberg, M.J. Abuzzahab, The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. J. Am. Med. Assoc. Pediatr. 167, 355–360 (2013)
Gallwitz, B, Extra-pancreatic effects of incretin-based therapies. Endocrine. (2014) [Epub ahead of print]
C. Verdich, A. Flint, J.P. Gutzwiller, E. Naslund, C. Beglinger, P.M. Hellstrom, S.J. Long, L.M. Morgan, J.J. Holst, A. Astrup, A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 86, 4382–4389 (2001)
A.S. Kelly, A.M. Metzig, K.D. Rudser, A.K. Fitch, C.K. Fox, B.M. Nathan, M.M. Deering, B.L. Schwartz, M.J. Abuzzahab, L.M. Gandrud, A. Moran, C.J. Billington, Schwarzenberg, SJ:Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity 20, 364–370 (2012)
R.A. DeFronzo, R.E. Ratner, J. Han, D.D. Kim, M.S. Fineman, A.D. Baron, Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28, 1092–1100 (2005)
M. Davies, R. Pratley, M. Hammer, A.B. Thomsen, R. Cuddihy, Liraglutide improves treatment satisfaction in people with Type 2 diabetes compared with sitagliptin, each as an add on to metformin. Diabet. Med. 28, 333–337 (2011)
J. Rosenstock, L.J. Klaff, S. Schwartz, J. Northrup, J.H. Holcombe, K. Wilhelm, M. Trautmann, Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care 33, 1173–1175 (2010)
K. Elkind-Hirsch, O. Marrioneaux, M. Bhushan, D. Vernor, R. Bhushan, Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 2670–2678 (2008)
S.M. Jensterle, T. Kocjan, M. Pfeifer, N.A. Kravos, A. Janez, Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur. J. Endocrinol. 170, 451–459 (2014)
H.F. Escobar-Morreale, Surgical management of metabolic dysfunction in PCOS. Steroids 77, 312–316 (2012)
H.F. Escobar-Morreale, J.I. Botella-Carretero, F. Alvarez-Blasco, J. Sancho, J.L. San Millan, The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 90, 6364–6369 (2005)
G.M. Eid, D.R. Cottam, L.M. Velcu, S.G. Mattar, M.T. Korytkowski, G. Gosman, P. Hindi, P.R. Schauer, Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 1, 77–80 (2005)
R. Morinigo, V. Moize, M. Musri, A.M. Lacy, S. Navarro, J.L. Marin, S. Delgado, R. Casamitjana, J. Vidal, Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab. 91, 1735–1740 (2006)
S. Evans, Z. Pamuklar, J. Rosko, P. Mahaney, N. Jiang, C. Park, A. Torquati, Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction. Surg. Endosc. 26, 1086–1094 (2012)
M.B. Mumphrey, L.M. Patterson, H. Zheng, H.R. Berthoud, Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil. 25, e70–e79 (2013)
J. Korner, M. Bessler, L.J. Cirilo, I.M. Conwell, A. Daud, N.L. Restuccia, S.L. Wardlaw, Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J. Clin. Endocrinol. Metab. 90, 359–365 (2005)
C.W. le Roux, R. Welbourn, M. Werling, A. Osborne, A. Kokkinos, A. Laurenius, H. Lonroth, L. Fandriks, M.A. Ghatei, S.R. Bloom, T. Olbers, Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 246, 780–785 (2007)
C.N. Ochner, E. Stice, E. Hutchins, L. Afifi, A. Geliebter, J. Hirsch, J. Teixeira, Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 209, 128–135 (2012)
Acknowledgments
This work was supported by grant from the Shanghai Jiaotong University, School of Medicine, Science and Technology Fund (Grant No. 12XJ10015).
Disclosure
There are no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, J., Lin, T.C. & Liu, W. Gastrointestinal hormones and polycystic ovary syndrome. Endocrine 47, 668–678 (2014). https://doi.org/10.1007/s12020-014-0275-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0275-1