Abstract
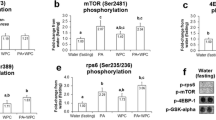
Data on the independent and potential combined effects of acid–base balance and vitamin D status on muscle mass and metabolism are lacking. We investigated whether alkali supplementation with potassium bicarbonate (KHCO3), with or without vitamin D3 (±VD3), alters urinary nitrogen (indicator of muscle proteolysis), muscle fiber cross-sectional area (FCSA), fiber number (FN), and anabolic (IGF-1, Akt, p70s6k) and catabolic (FOXO3a, MURF1, MAFbx) signaling pathways regulating muscle mass. Thirty-six, 20-month-old, Fischer 344/Brown-Norway rats were randomly assigned in a 2 × 2 factorial design to one of two KHCO3-supplemented diets (±VD3) or diets without KHCO3 (±VD3) for 12 weeks. Soleus, extensor digitorum longus (EDL), and plantaris muscles were harvested at 12 weeks. Independent of VD3 group, KHCO3 supplementation resulted in 35 % lower mean urinary nitrogen to creatinine ratio, 10 % higher mean type I FCSA (adjusted to muscle weight), but no statistically different mean type II FCSA (adjusted to muscle weight) or FN compared to no KHCO3. Among VD3-replete rats, phosphorylated-Akt protein expression was twofold higher in the KHCO3 compared to no KHCO3 groups, but this effect was blunted in rats on VD3-deficient diets. Neither intervention significantly affected serum or intramuscular IGF-1 expression, p70s6k or FOXO3a activation, or MURF1 and MAFbx gene expression. These findings provide support for alkali supplementation as a promising intervention to promote preservation of skeletal muscle mass, particularly in the setting of higher vitamin D status. Additional research is needed in defining the muscle biological pathways that are being targeted by alkali and vitamin D supplementation.



Similar content being viewed by others
References
J.F. Aloia, D.M. McGowan, A.N. Vaswani, P. Ross, S.H. Cohn, Relationship of menopause to skeletal and muscle mass. Am. J. Clin. Nutr. 53, 1378–1383 (1991)
V.A. Hughes, W.R. Frontera, R. Roubenoff, W.J. Evans, M.A. Singh, Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am. J. Clin. Nutr. 76, 473–481 (2002)
J. Vormann, T. Remer, Dietary, metabolic, physiologic, and disease-related aspects of acid-base balance: foreword to the contributions of the second International Acid-Base Symposium. J. Nutr. 138, 413S–414S (2008)
T. Remer, F. Manz, Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 95, 791–797 (1995)
R.D. Lindeman, J. Tobin, N.W. Shock, Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc. 33, 278–285 (1985)
B. Dawson-Hughes, S.S. Harris, L. Ceglia, Alkaline diets favor lean tissue mass in older adults. Am. J. Clin. Nutr. 87, 662–665 (2008)
M.K. Abramowitz, T.H. Hostetter, M.L. Melamed, Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am. J. Kidney Dis. 58, 29–38 (2011)
B. Williams, E. Layward, J. Walls, Skeletal muscle degradation and nitrogen wasting in rats with chronic metabolic acidosis. Clin. Sci. (Lond.) 80, 457–462 (1991)
R.C. May, R.A. Kelly, W.E. Mitch, Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J. Clin. Invest. 77, 614–621 (1986)
W.W. Souba, R.J. Smith, D.W. Wilmore, Glutamine metabolism by the intestinal tract. J. Parenter. Enteral Nutr. 9, 608–617 (1985)
W.G. Guder, D. Haussinger, W. Gerok, Renal and hepatic nitrogen metabolism in systemic acid base regulation. J. Clin. Chem. Clin. Biochem. 25, 457–466 (1987)
W.E. Mitch, Cellular mechanisms of catabolism activated by metabolic acidosis. Blood Purif. 13, 368–374 (1995)
J.L. Bailey, B. Zheng, Z. Hu, S.R. Price, W.E. Mitch, Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J. Am. Soc. Nephrol. 17, 1388–1394 (2006)
L. Frassetto, R.C. Morris Jr, A. Sebastian, Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J. Clin. Endocrinol. Metab. 82, 254–259 (1997)
L. Ceglia, S.S. Harris, S.A. Abrams, H.M. Rasmussen, G.E. Dallal, B. Dawson-Hughes, Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J. Clin. Endocrinol. Metab. 94, 645–653 (2009)
B. Dawson-Hughes, C. Castaneda-Sceppa, S.S. Harris, N.J. Palermo, G. Cloutier, L. Ceglia, G.E. Dallal, Impact of supplementation with bicarbonate on lower-extremity muscle performance in older men and women. Osteoporos. Int. 21, 1171–1179 (2010)
R. Smith, G. Stern, Myopathy, osteomalacia and hyperparathyroidism. Brain 90, 593–602 (1967)
M. Visser, D.J. Deeg, P. Lips, Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 88, 5766–5772 (2003)
M.B. Snijder, N.M. van Schoor, S.M. Pluijm, R.M. van Dam, M. Visser, P. Lips, Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J. Clin. Endocrinol. Metab. 91, 2980–2985 (2006)
J.W. Prineas, A.S. Mason, R.A. Henson, Myopathy in metabolic bone disease. Br. Med. J. 1, 1034–1036 (1965)
G.D. Schott, M.R. Wills, Muscle weakness in osteomalacia. Lancet 1, 626–629 (1976)
H. Glerup, K. Mikkelsen, L. Poulsen, E. Hass, S. Overbeck, H. Andersen, P. Charles, E.F. Eriksen, Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calc. Tiss. Int. 66, 419–424 (2000)
D. Seigfried, J. Arruda, N. Kurtzman, Influence of vitamin D on bicarbonate reabsorption, in Phosphate Metabolism, ed. by S.G. Massry (Plenum Press, New York and London, 1978), pp. 395–404
R.A. Peraino, E. Ghafary, D. Rouse, B.J. Stinebaugh, W.N. Suki, Effect of 25-hydroxycholecalciferol on renal handling of sodium, calcium, and phosphate during bicarbonate infusion. Miner. Electrolyte Metab. 1, 321–329 (1978)
H. Kawashiwa, J.A. Kraut, K. Kurokawa, Metabolic acidosis suppresses 25-hydroxyvitamin in D3–1alpha-hydroxylase in the rat kidney. Distinct site and mechanism of action. J Clin Invest 70, 135–140 (1982)
S.W. Lee, J. Russell, L.V. Avioli, 25-hydroxycholecalciferol to 1,25-dihydroxycholechalciferol: conversion impaired by systemic metabolic acidosis. Science 175, 994–996 (1977)
P.G. Reeves, F.H. Nielsen, G.C. Fahey Jr, AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123, 1939–1951 (1993)
J. Mardon, V. Habauzit, A. Trzeciakiewicz, M.J. Davicco, P. Lebecque, S. Mercier, J.C. Tressol, M.N. Horcajada, C. Demigne, V. Coxam, Long-term intake of a high-protein diet with or without potassium citrate modulates acid-base metabolism, but not bone status, in male rats. J. Nutr. 138, 718–724 (2008)
L. Doyle, K.D. Cashman, The effect of nutrient profiles of the Dietary Approaches to Stop Hypertension (DASH) diets on blood pressure and bone metabolism and composition in normotensive and hypertensive rats. Br. J. Nutr. 89, 713–724 (2003)
A.A. Welch, A. Mulligan, S.A. Bingham, K.T. Khaw, Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br. J. Nutr. 99, 1335–1343 (2008)
J.L. Greger, S.M. Kaup, A.R. Behling, Calcium, magnesium and phosphorus utilization by rats fed sodium and potassium salts of various inorganic anions. J. Nutr. 121, 1382–1388 (1991)
Z. Ren, M. Pae, M. Dao, D. Smith, S. Meydani, D. Wu, Dietary supplementation with tocotrienols enhances immune function in C57BL/6 mice. J. Nutr. 140, 1335–1341 (2010)
M.H. Brooke, K.K. Kaiser, Muscle fiber types: how many and what kind? Arch. Neurol. 23, 369–379 (1970)
L. Ceglia, S. Niramitmahapanya, L.L. Price, S.S. Harris, R.A. Fielding, B. Dawson-Hughes, An evaluation of the reliability of muscle fiber cross-sectional area and fiber number measurements in rat skeletal muscle. Biol. Proced. Online 15, 6 (2013)
G.B. Forbes, G.J. Bruining, Urinary creatinine excretion and lean body mass. Am. J. Clin. Nutr. 29, 1359–1366 (1976)
R. Swaminathan, J.A. Bradley, G.H. Hill, D.B. Morgan, The nitrogen to creatinine ratio in untimed samples of urine as an index of protein catabolism after surgery. Postgrad. Med. J. 55, 858–861 (1979)
S.J. Wassner, J.B. Li, A. Sperduto, M.E. Norman, Vitamin D deficiency, hypocalcemia, and increased skeletal muscle degradation in rats. J. Clin. Invest. 72, 102–112 (1983)
H.A. Bischoff-Ferrari, T. Dietrich, E.J. Orav, F.B. Hu, Y. Zhang, E.W. Karlson, B. Dawson-Hughes, Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am. J. Clin. Nutr. 80, 752–758 (2004)
S. Schiaffino, C. Mammucari, Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle 1, 4 (2011)
H.A. Franch, S. Raissi, X. Wang, B. Zheng, J.L. Bailey, S.R. Price, Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. Am. J. Physiol. Renal Physiol. 287, F700–F706 (2004)
H.N. Hulter, Effects and interrelationships of PTH, Ca2+, vitamin D, and Pi in acid-base homeostasis. Am. J. Physiol. 248, F739–F752 (1985)
N. Buitrago, R. Arango, Boland, 1alpha,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J. Cell. Biochem. 113, 1170–1181 (2012)
Q.G. Zhou, F.F. Hou, Z.J. Guo, M. Liang, G.B. Wang, X. Zhang, 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab. Res. Rev. 24, 459–464 (2008)
Acknowledgments
The authors thank the Comparative Biology Unit and the Nutrition Evaluation Laboratory at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University for their help with the study. The research study was supported by the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679). Additional support was provided by the Tufts Clinical and Translational Science Institute Grant (UL1 RR025752) from the National Center for Research Resources and the Gerald J. and Dorothy R. Friedman Foundation. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources. This material is also based upon work supported by the USDA, Agricultural Research Service, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Conflict of interest
The authors LC, DAR, RP, LLP, SSH, DS, RAF, and BDH have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was performed at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111.
Rights and permissions
About this article
Cite this article
Ceglia, L., Rivas, D.A., Pojednic, R.M. et al. Effects of alkali supplementation and vitamin D insufficiency on rat skeletal muscle. Endocrine 44, 454–464 (2013). https://doi.org/10.1007/s12020-013-9976-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-9976-0