Abstract
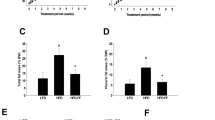
Fenofibrate is a peroxisome proliferator-activated receptor-α that has been clinically used to treat dyslipidemia and insulin resistance. To better understand the molecular mechanisms underlying fenofibrate action, we investigated whether fenofibrate affects serum levels of vaspin, an adipocytokine that has recently been shown to link obesity and insulin resistance. Fenofibrate treatment significantly increased serum vaspin levels of dyslipidemic patients, which correlated with reduced body weight and increased insulin sensitivity. To elucidate the biochemical mechanisms of fenofibrate action, we investigated the effect of fenofibrate on vaspin mRNA and protein expressions in obese rats. Fenofibrate greatly increased vaspin mRNA and protein levels in visceral adipose tissue consisting of retroperitoneal, mesenteric, and periepididymal adipose tissue but not in the subcutaneous adipose tissue, which correlated with increased serum vaspin levels and increased insulin sensitivity in obese rats. Consistent with a direct effect on vaspin expression, fenofibrate treatment significantly increased the mRNA and protein expression levels of vaspin in 3T3-L1 adipocytes. Together, our results demonstrate for the first time that fenofibrate upregulates vaspin expression in dyslipidemic human subjects and suggest that upregulation of vaspin expression in adipocytes may provide a mechanism by which fenofibrate improves insulin sensitivity in dyslipidemic patients.








Similar content being viewed by others
References
J.C. Fruchart, P. Duriez, Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc) 42, 39–64 (2006)
C.V. Iannucci, D. Capoccia, M. Calabria, F. Leonetti, Metabolic syndrome and adipose tissue: new clinical aspects and therapeutic targets. Curr. Pharm. Des. 13, 2148–2168 (2007)
F.P. Mancini, A. Lanni, L. Sabatino, M. Moreno, A. Giannino, F. Contaldo, V. Colantuoni, F. Goglia, Fenofibrate prevents and reduces body weight gain and adiposity in diet-induced obese rats. FEBS Lett. 491, 154–158 (2001)
E.K. Naderali, S. Fatani, M. Telles, L. Hunter, The effects of physiological and pharmacological weight loss on adiponectin and leptin mRNA levels in the rat epididymal adipose tissue. Eur. J. Pharmacol. 579, 433–438 (2008)
H. Wu, L. Wei, Y. Bao, J. Lu, P. Huang, Y. Liu, W. Jia, K. Xiang, Fenofibrate reduces serum retinol-binding protein-4 by suppressing its expression in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 296, E628–E634 (2009)
K. Hida, J. Wada, J. Eguchi, H. Zhang, M. Baba, A. Seida, I. Hashimoto, T. Okada, A. Yasuhara, A. Nakatsuka, K. Shikata, S. Hourai, J. Futami, E. Watanabe, Y. Matsuki, R. Hiramatsu, S. Akagi, H. Makino, Y.S. Kanwar, Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 102, 10610–10615 (2005)
N. Klöting, P. Kovacs, M. Kern, J.T. Heiker, M. Fasshauer, M.R. Schön, M. Stumvoll, A.G. Beck-Sickinger, M. Blüher, Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 54, 1819–1823 (2011)
N. Klöting, J. Berndt, S. Kralisch, P. Kovacs, M. Fasshauer, M.R. Schön, M. Stumvoll, M. Blüher, Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 339, 430–436 (2006)
K. Kempf, B. Rose, T. Illig, W. Rathmann, K. Strassburger, B. Thorand, C. Meisinger, H.E. Wichmann, C. Herder, C. Vollmert, Vaspin (SERPINA12) genotypes and risk of type 2 diabetes: results from the MONICA/KORA studies. Exp. Clin. Endocrinol. Diabetes 118, 184–189 (2010)
B.S. Youn, N. Klöting, J. Kratzsch, N. Lee, J.W. Park, E.S. Song, K. Ruschke, A. Oberbach, M. Fasshauer, M. Stumvoll, M. Blüher, Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 57, 372–377 (2008)
B.L. Tan, D. Heutling, J. Chen, S. Farhatullah, R. Adya, S.D. Keay, C.R. Kennedy, H. Lehnert, H.S. Randeva, Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 57, 1501–1507 (2008)
N.E. Gulcelik, J. Karakaya, A. Gedik, A. Usman, A. Gurlek, Serum vaspin levels in type 2 diabetic women in relation to microvascular complications. Eur. J. Endocrinol. 160, 65–70 (2009)
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive Summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001)
K.G. Alberti, P.Z. Zimmet, Definition, diagnosis and classification of diabetes mellitus and its complications. 1. Diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998)
A. Katz, S.S. Nambi, K. Mather, A.D. Baron, D.A. Follmann, G. Sullivan, M.J. Quon, Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 85, 2402–2410 (2000)
B. Murali, U.M. Upadhyaya, R.K. Goyal, Effect of chronic treatment with Enicostemma littorale in non-insulin-dependent diabetic rats. J. Ethnopharmacol. 81, 199–204 (2002)
Y.M. Wang, W.P. Wang, L.P. Wang, Q.H. Lü, X.H. Zhou, Calorie control increased vaspin levels of serum and periepididymal adipose tissue in diet-induced obese rats in association with serum free fatty acid and tumor necrosis factor alpha. Chin. Med. J. (Engl) 123, 936–941 (2010)
S. Suleymanoglu, E. Tascilar, O. Pirgon, S. Tapan, C. Meral, A. Abaci, Vaspin and its correlation with insulin sensitivity indices in obese children. Diabetes Res. Clin. Pract. 84, 325–328 (2009)
A. Hiuge, A. Tenenbaum, N. Maeda, M. Benderly, M. Kumada, E.Z. Fisman, D. Tanne, Z. Matas, T. Hibuse, K. Fujita, H. Nishizawa, Y. Adler, M. Motro, S. Kihara, I. Shimomura, S. Behar, T. Funahashi, Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin Level. Arterioscler. Thromb. Vasc. Biol. 27, 635–641 (2007)
J.C. Adkins, D. Faulds, Micronised fenofibrate: a review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidaemia. Drugs 54, 615–633 (1997)
N. Vu-Dac, K. Schoonjans, V. Kosykh, J. Dallongeville, J.C. Fruchart, B. Staels, J. Auwerx, Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J. Clin. Invest. 96, 741–750 (1995)
C.R. Gonzalez, J.E. Caminos, M.J. Vazquez, M.F. Garces, L.A. Cepeda, A. Angel, A.C. González, M.E. García-Rendueles, S. Sangiao-Alvarellos, M. López, S.B. Bravo, R. Nogueiras, C. Diéguez, Regulation of visceral adipose tissue-derived serine protease inhibitor by nutritional status, metformin, gender and pituitary factors in rat white adipose tissue. J. Physiol. 587, 3741–3750 (2009)
J.N. Fain, B. Buehrer, S.W. Bahouth, D.S. Tichansky, A.K. Madan, Comparison of messenger RNA distribution for 60 proteins in fat cells vs the nonfat cells of human omental adipose tissue. Metabolism 57, 1005–1015 (2008)
C. Fernandes-Santos, R.E. Carneiro, L. de Souza, M.B. Mendonca, C.A. Aguila, Mandarim-de-Lacerda, Pan-PPAR agonist beneficial effects in overweight mice fed a high-fat high-sucrose diet. Nutrition 25, 818–827 (2009)
S. Jeong, M. Yoon, Fenofibrate inhibits adipocyte hypertrophy and insulin resistance by activating adipose PPARalpha in high fat diet-induced obese mice. Exp. Mol. Med. 41, 397–405 (2009)
A. Golpaie, N. Tajik, F. Masoudkabir, Z. Karbaschian, M. Talebpour, M. Hoseini, M.J. Hosseinzadeh-Attar, Short-term effect of weight loss through restrictive bariatric surgery on serum levels of vaspin in morbidly obese subjects. Eur. Cytokine Netw. 22, 181–186 (2011)
M. Blüher, A. Rudich, N. Klöting, R. Golan, Y. Henkin, E. Rubin, D. Schwarzfuchs, Y. Gepner, M.J. Stampfer, M. Fiedler, J. Thiery, M. Stumvoll, I. Shai, Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care 35, 342–349 (2012)
M. Blüher, Vaspin in obesity and diabetes: pathophysiological and clinical significance. Endocrine 41, 176–182 (2012)
J. Wada, Vaspin: a novel serpin with insulin-sensitizing effects. Epert Opin. Investig. Drugs 17, 327–333 (2008)
Q. Li, R. Chen, J. Moriya, J. Yamakawa, H. Sumino, T. Kanda, T. Takahashi, A novel adipocytokine visceral adipose tissue-derived serine protease inhibitor (Vaspin), and obesity. J. Int. Med. Res. 36, 625–629 (2008)
J.A. Lee, H.S. Park, Y.S. Song, Y.J. Jang, J.H. Kim, Y.J. Lee, Y.S. Heo, Relationship between vaspin gene expression and abdominal fat distribution of Korean women. Endocr. J. 8, 639–646 (2011)
M. Pardo, A. Roca-Rivada, L.M. Seoane, F.F. Casanueva, Obesidomics: contribution of adipose tissue secretome analysis to obesity research. Endocrine 41, 374–383 (2012)
Acknowledgments
This study was supported by the national TCM clinical research base of special foundation (No. JDZX2012005) and the Ministry of Education Foundation of Anhui Province (No. KJ2011A161), PR China. We thank Wenping Wang, Liping Wang, Qihuan Lü, and Xiaohui Zhou for their scientific input and assistance in various aspects of these studies.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mingwei Chen and Datong Deng have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Chen, M., Deng, D., Fang, Z. et al. Fenofibrate increases serum vaspin by upregulating its expression in adipose tissue. Endocrine 45, 409–421 (2014). https://doi.org/10.1007/s12020-013-0023-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-0023-y