Abstract
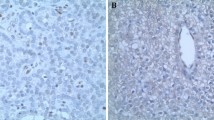
Aberrant accumulation of β-catenin plays an important role in a variety of human neoplasms. This can be caused by stabilizing mutation of β-catenin (CTNNB1, exon 3) or by mutation or deregulated expression of other components of the WNT/β-catenin signaling pathway. Accumulation of non-phosphorylated active β-catenin has been reported to commonly occur in parathyroid adenomas from patients with primary hyperparathyroidism (pHPT), either due to the aberrantly spliced internally truncated WNT receptor LRP5 (LRP5Δ) or to a stabilizing mutation of β-catenin. The S37A mutation was reported to occur in 7.3 % in a single study of parathyroid adenomas, while in other studies no stabilizing mutations of β-catenin exon 3 were identified. The aim of this study was to determine the mutational frequency of the CTNNB1 gene, specifically exon 3 in a large series of parathyroid adenomas. One hundred and eighty sporadic parathyroid adenomas were examined for mutations in exon 3 of CTNNB1 by direct DNA sequencing, utilizing previously published primer sequences. The mutation S33C (TCT>TGT) was detected by direct-DNA sequencing of PCR fragments in 1 out of 180 sporadic parathyroid adenomas (0.68 %). Like serine 37, mutations of serine 33 have been reported in many neoplasms with resulting β-catenin stabilization, enhanced transcription, and oncogenic activities. Immunohistochemical analysis revealed an overexpression of the β-catenin protein in the lone mutant tumor. Taking also previous studies into account we conclude that activating mutations of the regulatory GSK-3β phosphorylation sites serine 33 and 37, encoded by CTNNB1 exon 3, rarely occur in parathyroid adenomas from patients with pHPT.


Similar content being viewed by others
References
H. Heath 3rd, S.F. Hodgson, M.A. Kennedy, Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. N. Engl. J. Med. 302, 189–193 (1980)
E. Lundgren, E. Szabo, S. Ljunghall, R. Bergstrom, L. Holmberg, J. Rastad, Population based case-control study of sick leave in postmenopausal women before diagnosis of hyperparathyroidism. BMJ 317, 848–851 (1998)
S.J. Silverberg, E.M. Lewiecki, L. Mosekilde, M. Peacock, M.R. Rubin, Presentation of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J. Clin. Endocrinol. Metab. 94, 351–365 (2009)
C.A. Verdonk, A.J. Edis, Parathyroid “double adenomas”: fact of fiction? Surgery 90, 523–526 (1981)
P.K. Lee, S.L. Jarosek, B.A. Virnig, M. Evasovich, T.M. Tuttle, Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer 109, 1736–1741 (2007)
L.E. Tisell, S. Carlsson, M. Fjalling, G. Hansson, S. Lindberg, L.M. Lundberg, A. Oden, Hyperparathyroidism subsequent to neck irradiation risk factors. Cancer 56, 1529–1533 (1985)
G. Westin, P. Bjorklund, G. Akerstrom, Molecular genetics of parathyroid disease. World J. Surg. 33, 2224–2233 (2009)
T. Carling, P. Correa, O. Hessman, J. Hedberg, B. Skogseid, D. Lindberg, J. Rastad, G. Westin, G. Akerstrom, Parathyroid MEN1 gene mutations in relation to clinical characteristics of nonfamilial primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 83, 2960–2963 (1998)
F. Farnebo, B.-T. Teh, S. Kytölä, A. Svensson, C. Phelan, K. Sandelin, N.W. Thompson, A. Höög, G. Weber, L.-O. Farnebo, C. Larsson, Alterations of the MEN1 gene in sporadic parathyroid tumors. J. Clin. Endocrinol. Metab. 83, 2627–2630 (1998)
A. Arnold, H.G. Kim, Clonal loss of one chromosome 11 in a parathyroid adenoma. J. Clin. Endocrinol. Metab. 69, 496–499 (1989)
M. Shtutman, J. Zhurinsky, I. Simcha, C. Albanese, M. D’Amico, R. Pestell, A. Ben-Ze’ev, The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96, 5522–5527 (1999)
P. Bjorklund, D. Lindberg, G. Akerstrom, G. Westin, Stabilizing mutation of CTNNB1/beta-catenin and protein accumulation analyzed in a large series of parathyroid tumors of Swedish patients. Mol. Cancer 7, 53 (2008)
P. Bjorklund, G. Akerstrom, G. Westin, Accumulation of nonphosphorylated beta-catenin and c-myc in primary and uremic secondary hyperparathyroid tumors. J. Clin. Endocrinol. Metab. 92, 338–344 (2007)
P. Bjorklund, G. Akerstrom, G. Westin, Activated beta-catenin in the novel human parathyroid tumor cell line sHPT-1. Biochem. Biophys. Res. Commun. 352, 532–536 (2007)
J. Costa-Guda, A. Arnold, Absence of stabilizing mutations of beta-catenin encoded by CTNNB1 exon 3 in a large series of sporadic parathyroid adenomas. J. Clin. Endocrinol. Metab. 92, 1564–1566 (2007)
S. Ikeda, Y. Ishizaki, Y. Shimizu, M. Fujimori, Y. Ojima, M. Okajima, K. Sugino, T. Asahara, Immunohistochemistry of cyclin D1 and beta-catenin, and mutational analysis of exon 3 of beta-catenin gene in parathyroid adenomas. Int. J. Oncol. 20, 463–466 (2002)
S. Semba, R. Kusumi, T. Moriya, H. Sasano, Nuclear accumulation of B-catenin in human endocrine tumors: association with Ki-67 (MIB-1) proliferative activity. Endocr. Pathol. 11, 243–250 (2000)
P. Bjorklund, G. Akerstrom, G. Westin, An LRP5 receptor with internal deletion in hyperparathyroid tumors with implications for deregulated WNT/beta-catenin signaling. PLoS Med 4, e328 (2007)
F. Haglund, A. Andreasson, I.L. Nilsson, A. Hoog, C. Larsson, C.C. Juhlin, Lack of S37A CTNNB1/beta-catenin mutations in a Swedish cohort of 98 parathyroid adenomas. Clin. Endocrinol. (Oxf) 73, 552–553 (2010)
P. Polakis, The oncogenic activation of beta-catenin. Curr. Opin. Genet. Dev. 9, 15–21 (1999)
V. Guarnieri, F. Baorda, C. Battista, M. Bisceglia, T. Balsamo, E. Gruppioni, M. Fiorentino, L.A. Muscarella, M. Coco, R. Barbano, S. Corbetta, A. Spada, D.E. Cole, L. Canaff, G.N. Hendy, M. Carella, A. Scillitani. A rare S33C mutation of CTNNB1 encoding β-catenin in a parathyroid adenoma found in an Italian primary hyperparathyroid cohort. Endocrine (2011) doi:10.1007/s12020-011-9558-y
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starker, L.F., Fonseca, A., Åkerström, G. et al. Evidence of a stabilizing mutation of β-catenin encoded by CTNNB1 exon 3 in a large series of sporadic parathyroid adenomas. Endocrine 42, 612–615 (2012). https://doi.org/10.1007/s12020-012-9690-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9690-3