Abstract
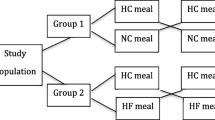
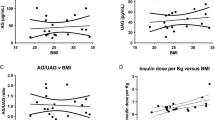
Ghrelin, an orexigenic hormone, may be involved in the etiology of obesity. African Americans (AA) experience higher obesity rates than European Americans (EA), but it is unclear whether ghrelin differs with ethnicity. This study was designed to compare ghrelin concentrations between overweight AA and EA adults in a post absorptive state, in response to a standard meal, and after 8-week habituation to diets of differing macronutrient profiles. Sixty-one overweight men and women (31 EA and 30 AA) were assigned to either a higher-carbohydrate/lower-fat diet (55 % CHO, 18 % PRO, 27 % FAT) or a lower-carbohydrate/higher-fat diet (43 % CHO, 18 % PRO, 39 % FAT) for 8 weeks. At baseline and week 8, participants ingested a standard liquid mixed meal. Blood was sampled before the meal and serially after ingestion to measure total ghrelin and insulin. Hunger was assessed with a visual analog scale. Composite scores for ghrelin, insulin, and hunger were calculated as area under the curve (AUC), and ghrelin suppression was calculated as the change from fasting concentration. Fasting ghrelin and ghrelin AUC were higher among EA at baseline and week 8 (p < 0.001), and these differences were not affected by diet habituation. Despite greater postprandial ghrelin suppression, EA displayed greater hunger immediately following the test meal (p < 0.05). Overweight EA displayed higher circulating ghrelin and greater ghrelin suppression compared to AA. Further study is warranted to explore the physiological basis for these ethnic differences and to determine whether they may relate to higher obesity rates among AA.





Similar content being viewed by others
References
H. Ueno, T. Shiiya, M. Nakazato, Translational research of ghrelin. Ann. N. Y. Acad. Sci. 1200, 120–127 (2010)
K.M. Flegal, M.D. Carroll, C.L. Ogden, L.R. Curtin, Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 (2010)
M. Patterson, S.R. Bloom, J.V. Gardiner, Ghrelin and appetite control in humans—potential application in the treatment of obesity. Peptides 11, 2290–2294 (2011)
D. Perez-Tilve, R. Nogueiras, F. Mallo, S.C. Benoit, M. Tschoep, Gut hormones ghrelin, PYY, and GLP-1 in the regulation of energy balance [corrected] and metabolism. Endocrine 29, 61–71 (2006)
M. Möhlig, J. Spranger, B. Otto, M. Ristow, M. Tschöp, A.F. Pfeiffer, Euglycemic hyperinsulinemia, but not lipid infusion, decreases circulating ghrelin levels in humans. J. Endocrinol. Invest. 25, RC36–RC38 (2002)
M.F. Saad, B. Bernaba, C.M. Hwu et al., Insulin regulates plasma ghrelin concentration. J. Clin. Endocrinol. Metab. 87, 3997–4000 (2002)
D.E. Flanagan, M.L. Evans, T.P. Monsod et al., The influence of insulin on circulating ghrelin. Am. J. Physiol. Endocrinol. Metab. 284, E313–E316 (2003)
J. Albu, A. Kovera, L. Allen et al., Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am. J. Clin. Nutr. 82, 1210–1217 (2005)
P. Chandler-Laney, R.P. Phadke, W.M. Granger et al., Adiposity and beta-cell function: relationships differ with ethnicity and age. Obesity 18(11), 2086–2092 (2010)
B. Frazier, C.W. Hsiao, P. Deuster, M. Poth, African Americans and Caucasian Americans: differences in glucocorticoid-induced insulin resistance. Horm. Metab. Res. 42, 887–891 (2010)
F. Broglio, C. Gottero, A. Benso et al., Ghrelin and the endocrine pancreas. Endocrine 22, 19–24 (2003)
K. Foster-Schubert, J. Overduin, C. Prudom et al., Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 93, 1971–1979 (2008)
D. Tannous dit El Khoury, O. Obeid, S.T. Azar, N. Hwalla, Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann. Nutr. Metab. 50, 260–269 (2006)
J. Erdmann, R. Töpsch, F. Lippl, P. Gussmann, V. Schusdziarra, Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J. Clin. Endocrinol. Metab. 89, 3048–3054 (2004)
L.L. Goree, P. Chandler-Laney, A.C. Ellis, K. Casazza, W.M. Granger, B.A. Gower, Dietary macronutrient composition affects {beta} cell responsiveness but not insulin sensitivity. Am. J. Clin. Nutr. 94, 120–127 (2011)
B.A. Gower, L.L. Goree, P.C. Chandler-Laney, A.C. Ellis, K. Casazza, W.M. Granger, A higher-carbohydrate, lower-fat diet reduces fasting glucose concentration and improves β-cell function in individuals with impaired fasting glucose. Metabolism 61, 358–365 (2012)
J. Harris, F. Benedict, A Biometric Study of Human Basal Metabolism. Proc. Natl Acad. Sci. USA 4, 370–373 (1918)
A. Flint, A. Raben, J.E. Blundell, A. Astrup, Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 24, 38–48 (2000)
J.N. Matthews, D.G. Altman, M.J. Campbell, P. Royston, Analysis of serial measurements in medical research. BMJ 300, 230–235 (1990)
A. Salbe, M. Tschöp, A. DelParigi, C. Venti, P. Tataranni, Negative relationship between fasting plasma ghrelin concentrations and ad libitum food intake. J. Clin. Endocrinol. Metab. 89, 2951–2956 (2004)
M. Tschöp, C. Weyer, P.A. Tataranni, V. Devanarayan, E. Ravussin, M.L. Heiman, Circulating ghrelin levels are decreased in human obesity. Diabetes 50, 707–709 (2001)
S. Matsunaga-Irie, H. Ueshima, W.R. Zaky et al., Serum ghrelin levels are higher in Caucasian men than Japanese men aged 40–49 years. Diabetes Obes. Metab. 9, 591–593 (2007)
F. Bacha, S. Arslanian, Ghrelin and peptide YY in youth: are there race-related differences? J. Clin. Endocrinol. Metab. 91, 3117–3122 (2006)
K. Brownley, K. Light, K. Grewen, E. Bragdon, A. Hinderliter, S. West, Postprandial ghrelin is elevated in black compared with white women. J. Clin. Endocrinol. Metab. 89, 4457–4463 (2004)
J.P. Camiña, M.C. Carreira, D. Micic et al., Regulation of ghrelin secretion and action. Endocrine 22, 5–12 (2003)
M. Kojima, K. Kangawa, Ghrelin: more than endogenous growth hormone secretagogue. Ann. N. Y. Acad. Sci. 1200, 140–148 (2010)
K. Dezaki, H. Sone, T. Yada, Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol. Ther. 118, 239–249 (2008)
S. Seino, T. Shibasaki, K. Minami, Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J. Clin. Invest. 121, 2118–2125 (2011)
J.M. Jakicic, R.R. Wing, Differences in resting energy expenditure in African-American vs Caucasian overweight females. Int. J. Obes. Relat. Metab. Disord. 22, 236–242 (1998)
D. Gallagher, J. Albu, Q. He et al., Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am. J. Clin. Nutr. 83, 1062–1067 (2006)
R.P. Vincent, H. Ashrafian, C.W. le Roux, Mechanisms of disease: the role of gastrointestinal hormones in appetite and obesity. Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 268–277 (2008)
National Academy of Sciences. Institute of Medicine. Food and Nutrition Board, Dietary reference intakes: macronutrients. DRI table for carbohydrate, fiber, fat, fatty acids and protein (2005), http://www.nap.edu. Accessed 01 Sep 2011
Acknowledgments
This study was supported by the National Institute of Health and National Institute of Diabetes and Digestive and Kidney Disease (R01DK67538). Core laboratory support, nursing, and inpatient/outpatient facilities were provided by M01-RR-00032 (GCRC), UL1RR025777 (CTSA), P30-DK56336 (NORC), P60DK079626 (DRTC). The authors are appreciative of Betty Darnell and Suzanne Choquette for coordination of the GCRC Bionutrition kitchen and Maryellen Williams and Cindy Zeng for laboratory analyses.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trials registration
This study is registered on www.clinicaltrials.gov (ClinicalTrials.gov ID: NCT00726908).
Rights and permissions
About this article
Cite this article
Ellis, A.C., Chandler-Laney, P., Casazza, K. et al. Effects of habitual diet on ethnic differences in serum total ghrelin. Endocrine 42, 359–365 (2012). https://doi.org/10.1007/s12020-012-9667-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9667-2