Abstract
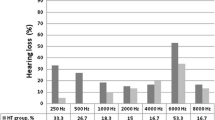
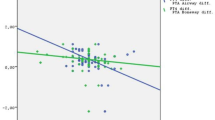
Hearing loss has commonly been reported in association with thyroid disorders and during treatment with propylthiouracil. The relationship between hyperthyroidism and the auditory system has not been previously investigated. The aim of this cross-sectional, case–control study was to investigate hearing loss in patients with Graves’ disease (GD). The study population consisted of patients with newly diagnosed GD and healthy controls. Pure tone audiometry at frequencies of 250, 500, 1000, 2000, 4000 and 8000 Hz, along with immittance measures including tympanometry and acoustic reflex tests, were performed in all participants. Twenty-two GD patients and 22 healthy controls consented to inclusion in the study. The differences between groups with regards to age and gender distribution were statistically insignificant (P = 0.567 and P = 0.757, respectively). The hearing thresholds of right and left ears were also similar in both groups (P > 0.05). When single-ear evaluations were taken into account (total of 44 ears for both groups), hearing thresholds in the GD group were significantly higher than healthy controls at all frequencies (P < 0.05). Following testing at the designated frequencies, the only significant effect of thyrotoxicosis was observed with frequencies of 4000 and 8000 Hz. The odds ratio for having hearing loss at a frequency of 8000 HZ associated with GD was 14.97 (95% confidence interval 4.03–55.64). In patients with GD, right and left pure tone audiometric findings at a frequency of 8000 Hz correlated positively with FT3, FT4 and negatively with TSH. Our results are highly suggestive of a decrease in hearing ability in patients with GD, particularly at high frequencies. Further studies are needed to help elucidate the mechanisms behind hearing loss which develops in association with GD.

Similar content being viewed by others
References
A. Rusch, L.C. Erway, D. Oliver, B. Wenstrom, D. Forrest, Thyroid hormone receptor β-dependent expression of a potassium conductance in inner hair cells at the onset of hearing. Proc. Natl. Acad. Sci. USA 95, 15758–15762 (1998)
M. Knipper, C. Bandtlow, L. Gestwa, I. Köpschall, K. Rochbock, B. Wiechers, H.P. Zenner, U. Zimmermann, Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nevre prior to cochlea function. Development 125, 3709–3718 (1998)
F. Brucker-Davis, M.C. Skarulis, A. Pikus, D. Ishizawar, M.A. Mastroianni, M. Koby, B.D. Weintraub, Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone. J. Clin. Endocrinol. Metab. 81, 2768–2772 (1996)
E.S. Goldey, L.S. Kehn, G.L. Rehnberg, K.M. Crofton, Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol. Appl. Pharmacol. 135, 67–76 (1995)
M. Knipper, C. Zinn, H. Maier, M. Praetorius, K. Rohbock, I. Köpschall, U. Zimmermann, Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J. Neurophysiol. 83, 3101–3112 (2000)
E.S. Goldey, L.S. Kehn, C. Lau, G.L. Rehnberg, K.M. Crofton, Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduced circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol. Appl. Pharmacol. 135, 77–88 (1995)
K.M. Crofton, D. Ding, R. Padich, M. Taylor, D. Henderson, Hearing loss following exposure during development to polychlorinated biphenyls: a cochlear site of action. Hear. Res. 144, 196–204 (2000)
E.S. Goldey, K.M. Crofton, Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol. Sci. 45, 94–105 (1998)
S.C. Bellman, A. Davies, P.W. Fuggle, D.B. Grant, I. Smith, Mild impairment of neuro-otological function in early treated congenital hypothyroidism. Arch. Dis. Child. 74, 215–218 (1996)
F. Debruyne, M. Vanderschueren-Lodeweychx, P. Bastijns, Hearing in congenital hypothyroidism. Audiology 22, 404–409 (1983)
F. Soriguer, M.C. Millón, R. Muñoz, I. Mancha, J.P. López Siguero, M.J. Martinez Aedo, R. Gómez-Huelga, M.J. Garriga, G. Martinez, I. Esteva, F.J. Tinahones, The auditory threshold in a school-age population is related to iodine intake and thyroid function. Thyroid 10, 991–999 (2000)
A.L. Werneck, L.C. Gurgel, L.M. de Mello, G.Q. de Albuquerque, Sudden sensorineural hearing loss: a case report supporting the immunologic theory. Arq. Neuropsiquiatr. 61, 1018–1022 (2003)
S. Thamprajamchit, C. Jariengprasert, R. Rajatanavin, Propylthiouracil-induced sensorineural hearing loss associated with antineutrophil cytoplasmic antibodies. Endocr. Pract. 10, 432–437 (2004)
M. Sano, N. Kitahara, R. Kunikata, Progressive bilateral sensorineural hearing loss induced by an antithyroid drug. ORL. J. Otorhinolaryngol. Relat. Spec. 66, 281–285 (2004)
U. Bandyopadhyay, K. Biswas, R.K. Banerjee, Extrathyroidal actions of antithyroid thionamides. Toxicol. Lett. 128, 117–127 (2002)
J.P. Bilezekian, J.N. Loeb, The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocrinol. Rev. 4, 378–388 (1983)
H. Spoendlin, W. Lichtensteiger, The adrenergic innervation of the labyrinth. Acta Otolaryngol. 61, 423–434 (1966)
E.P. Fowler Jr., A. Zeckel, Psychosomatic aspects of meniere’s disease. J. Am. Med. Assoc. 148, 1265–1268 (1952)
N. Jones, J. Fex, R.A. Altschuler, Tyrosine hydroxylase immunoreactivity identifies possible catecholaminergic fibers in the organ of Corti. Hear. Res. 30, 33–38 (1987)
R. Volpe, Immunology of the thyroid, in Autoimmune disease of the endocrine system, ed. by R. Volpe (CRC Press, Boca Raton, 1990), pp. 73–239
J.M. Heward, A. Allahabadia, J. Daykin, J. Carr-Smith, A. Daly, M. Armitage, P.M. Dodson, M.C. Sheppard, A.H. Barnett, J.A. Franklyn, S.C. Gough, Linkage disequilibrium between the human leukocyte antigen class II region of the major histocompatibility complex and Graves’ disease: replication using a population case control and family-based study. J. Clin. Endocrinol. Metab. 83, 3394–3397 (1998)
H. Uno, T. Sasazuki, H. Tamai, H. Matsumoto, Two major genes, linked to HLA and Gm, control susceptibility to Graves’ disease. Nature 292, 768–770 (1981)
D.C. Shields, S. Ratanachaiyavong, A.M. McGregor, A. Collins, N.E. Morton, Combined segregation and linkage analysis of Graves disease with a thyroid autoantibody diathesis. Am. J. Hum. Genet. 55, 540–554 (1994)
A. Eryilmaz, M. Dagli, H. Karabulut, F. Sivas Acar, E. Erkol Inal, C. Gocer, Evaluation of hearing loss in patients with ankylosing spondylitis. J. Laryngol. Otol. 121, 845–849 (2007)
M.J. Ruckenstein, Autoimmune inner ear disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2, 426–430 (2004)
J.P. Harris, Immunology of the inner ear. Response of the inner ear to antigen challenge. Otolaryngol. Head Neck Surg. 91, 18–23 (1983)
G. Mogi, H. Kawauchi, M. Suzuki, N. Sato, Inner ear immunology. Am. J. Otolaryngol. 6, 142–147 (1985)
J.P. Harris, Immunology of the inner ear. Ann. Otol. Rhinol. 93, 157–162 (1984)
J.P. Harris, N.K. Woolf, A.F. Ryan, Elaboration of systemic immunity following inner ear immunization. Am. J. Otolaryngol. 6, 148–152 (1985)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berker, D., Karabulut, H., Isik, S. et al. Evaluation of hearing loss in patients with Graves’ disease. Endocrine 41, 116–121 (2012). https://doi.org/10.1007/s12020-011-9515-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-011-9515-9