Abstract
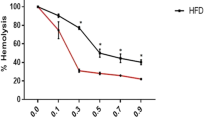
This study investigated the relation between erythrocyte osmotic fragility and oxidative stress and antioxidant state in primary hyperthyroidism induced experimental rats. Twenty-four Spraque–Dawley-type female rats weighing between 160 and 200 g were divided into two, as control (n = 10) and experimental (n = 12), groups. The experimental group animals have received tap water and L-Tiroksin (0.4 mg/100 g fodder) added standard fodder for 30 days to induce hyperthyroidism. Control group animals were fed tap water and standard fodder for the same period. Blood samples were drawn from the abdominal aorta of the rats under ether anesthesia. T3, T4, and TSH levels, osmotic fragility, malondialdehyde (MDA), superoxide dismutase, and glutathione levels were measured in the blood. There was a statistically significant deviation found in maximum and minimum osmotic hemolysis limit values of experimental group when compared to controls. The standard hemolytic increment curve of the hyperthyroid group shifted to the right when compared to control group’s curve. There was a statistically significant increase found in MDA and superoxide dismutase, but statistically a significant decrease was detected in glutathione levels in hyperthyroid group when compared to controls. As a result of our study, it may be concluded that hyperthyroidism may led to an increase in osmotic fragility of erythrocytes and this situation may possibly originate from the increased lipid peroxidation in hyperthyroidism.


Similar content being viewed by others
References
H. Sies, in Oxidative Stress, ed. by H. Sies (Academic Press, Orlando, 1985)
B. Halliwell, Lancet 344(10), 721–724 (1994)
G. Van Ginkel, A. Sevanian, Methods Enzymol. 233, 273–288 (1994)
E. Brzezinska-Slebodzinska, Acta Vet. Hung. 49, 413–419 (2001)
R.P.J. Hebbel, Lab. Clin. Med. 107, 401–404 (1986)
S.K. Jain, N. Mohandas, M.R. Clark, S.B. Shobel, Br. J. Haematol. 53, 247–252 (1983)
J.C. Debouzy, F. Fauvelle, H. Vezin, B. Brasme, Y. Chancerelle, Biochem. Pharmacol. 44, 1787–1793 (1992)
L. Mayer, Z. Romic, F. Skreb et al., Clin. Chem. Lab. Med. 42, 154–158 (2004)
K. Asayama, K. Dobashi, H. Hayashibe, Y. Megata, K. Kato, Endocrinology 21, 2112–2118 (1987)
T. Mano, R. Sinohara, Y. Sawai, N. Oda, Y. Nishida et al., J. Endocrinol. 147, 361–365 (1995)
R. Sinohara, T. Mano, A. Nagasaka, R. Hayashi, K. Uchimura, I. Nakano et al., J. Endocrinol. 164(1), 97–102 (2000)
P. Venditti, M. Balestrieri, S. Di Meo, T. De Leo, J. Endocrinol. 155, 151–157 (1997)
G. Andryskowski, T. Owczarek, Pol. Arch. Med. Wewn. 117(7), 285–289 (2007)
L. Mayer, Z. Romic, F. Skreb et al., Clin. Chem. Lab. Med. 42, 154–158 (2004)
R. Wilson, M. Chopra, H. Bradley et al., Clin. Endocrinol. 30, 429–433 (1989)
A. Seven, E. Tasan, H. Hatemi et al., Acta Med. Okayama 53, 27–30 (1999)
K. Komosinska-Vassev, K. Olczyk, E.J. Kucharz, C. Marcisz, K. Winsz-Sczotka, Clin. Chim. Acta 300, 107–117 (2000)
P. Mascio, M.E. Murphy, H. Sies, Am. J. Clin. Nutr. 53, 194–200 (1991)
E. Ademoglu, N. Ozbey, Y. Erbil et al., Eur. J. Intern. Med. 17, 545–550 (2006)
K. Kolanjiappan, K. Manoharan, Clin. Chim. Acta 326, 143–149 (2002)
T. Devasena, S. Lalitha, K. Padma, Med. Sci. Res. 27, 843–846 (1999)
O.A. Levander, S.O. Welsu, Life Sci. 28, 147–151 (1981)
S. Ozdemir, R. Yücel, N. Dariyerli, S. Toplan, M.C. Akyolcu, G. Yigit, H. Hatemi, Endocrine 30(2), 203–205 (2006)
J. Suess, D. Limenton, W. Dameshek, J.M.A. Dolloft, Blood 3, 1250–1303 (1954)
T.F. Slater, Methods Enzymol. 105, 283–293 (1984)
M.E. Anderson, in Enzymatic and Chemical Methods for Determination of Glutathione: Chemical, Biochemical and Medical Aspects, vol. A, ed. by D. Dolphin, R. Poulson, O. Avramovic (Wiley, New York, 1989)
C. Nebot, M. Moutet, P. Huet, J.Z. Xu, J.C. Yadan, J. Chaudiere, Anal. Biochem. 214, 442–451 (1993)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yücel, R., Özdemir, S., Darıyerli, N. et al. Erythrocyte osmotic fragility and lipid peroxidation in experimental hyperthyroidism. Endocr 36, 498–502 (2009). https://doi.org/10.1007/s12020-009-9251-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-009-9251-6