Abstract
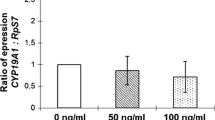
Polycystic ovary syndrome (PCOS) is characterized by the presence of hyperandrogenism and an increased follicular mass probably determined by deregulation of locally produced factors. Anti-Müllerian hormone (AMH) is a glycoprotein that inhibits follicular recruitment and determines the size of the follicular pool. To evaluate the role of androgens in the regulation of AMH expression in bovine granulosa cells from small follicles, granulosa cells from 3 to 4 mm follicles were isolated and incubated in basal culture media, or in media containing testosterone (T) at 10−5M, T 10−8M, or estradiol (E2) at 150 ng/ml for 48 h. AMH mRNA levels of these cells were determined using real-time PCR (RT PCR). AMH protein levels and E2 were determined in cell-conditioned media. A 3.4-fold decrease in AMH mRNA levels was observed in granulosa cells exposed to T 10−5M (P = 0.03, n = 5), but not in cells exposed to T 10−8M. AMH protein levels showed a 1.8-fold reduction in cell-conditioned media from cells exposed to T 10−5M (P = 0.01, n = 5), without significant changes in the group exposed to T 10−8M. Cells treated with E2 150 ng/ml showed no change in AMH protein levels. We propose that AMH expression is modulated by androgens in bovine granulosa cells from small follicles. Thus, it is possible to speculate that androgens, by inhibiting AMH expression, may promote follicle recruitment, increasing the early growing follicular pool. This new mechanism may have implications for the understanding of PCOS pathophysiology.





Similar content being viewed by others
References
L.J. Webber, S. Stubbs, J. Stark, G.H. Trew, R. Margara, K. Hardy, S. Franks, Formation and early development of follicles in the polycystic ovary. Lancet 362, 1017–1021 (2003)
S. Jonard, Y. Robert, C. Cortet-Rudelli, P. Pigny, C. Decanter, D. Dewailly, Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum. Reprod. 18, 598–603 (2003)
T. Sir-Petermann, E. Codner, M. Maliqueo, B. Echiburu, C. Hitschfeld, N. Crisosto, F. Perez-Bravo, S.E. Recabarren, F. Cassorla, Increased anti-Mullerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 91, 3105–3109 (2006)
N. Crisosto, E. Codner, M. Maliqueo, B. Echiburu, F. Sanchez, F. Cassorla, T. Sir-Petermann, Anti-Mullerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 92, 2739–2743 (2007)
S. Jonard, D. Dewailly, The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum. Reprod. Update 10, 107–117 (2004)
A. Gougeon, Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 17, 121–155 (1996)
D.H. Abbott, D.K. Barnett, C.M. Bruns, D.A. Dumesic, Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum. Reprod. Update 11, 357–374 (2005)
N. Josso, N. di Clemente, TGF-beta family members and gonadal development. Trends Endocrinol. Metab. 10, 216–222 (1999)
M.M. Lee, P.K. Donahoe, Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocr. Rev. 14, 152–164 (1993)
C. Weenen, J.S. Laven, A.R. Von Bergh, M. Cranfield, N.P. Groome, J.A. Visser, P. Kramer, B.C. Fauser, A.P. Themmen, Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 10, 77–83 (2004)
A.L. Durlinger, M.J. Gruijters, P. Kramer, B. Karels, H.A. Ingraham, M.W. Nachtigal, J.T. Uilenbroek, J.A. Grootegoed, A.P. Themmen, Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 143, 1076–1084 (2002)
A.L. Durlinger, M.J. Gruijters, P. Kramer, B. Karels, T.R. Kumar, M.M. Matzuk, U.M. Rose, F.H. de Jong, J.T. Uilenbroek, J.A. Grootegoed, A.P. Themmen, Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 142, 4891–4899 (2001)
N. di Clemente, B. Goxe, J.J. Remy, R.L. Cate, N. Josso, B. Vigier, R. Salesse, Inhibitory effect of AMH upon aromatase activity and LH receptors of granulosa cells of rat and porcine immature ovaries. Endocrine 2, 553–558 (1994)
L. Al-Attar, K. Noel, M. Dutertre, C. Belville, M.G. Forest, P.S. Burgoyne, N. Josso, R. Rey, Hormonal and cellular regulation of Sertoli cell anti-Mullerian hormone production in the postnatal mouse. J. Clin. Invest. 100, 1335–1343 (1997)
R. Rey, I. Lordereau-Richard, J.C. Carel, P. Barbet, R.L. Cate, M. Roger, J.L. Chaussain, N. Josso, Anti-mullerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. J. Clin. Endocrinol. Metab. 77, 1220–1226 (1993)
K.P. McNatty, D.M. Smith, A. Makris, C. DeGrazia, D. Tulchinsky, R. Osathanondh, I. Schiff, K.J. Ryan, The intraovarian sites of androgen and estrogen formation in women with normal and hyperandrogenic ovaries as judged by in vitro experiments. J. Clin. Endocrinol. Metab. 50, 755–763 (1980)
M. Mihm, A.C. Evans, Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women. Reprod. Domest. Anim. 43(Suppl 2), 48–56 (2008)
G.P. Adams, R. Jaiswal, J. Singh, P. Malhi, Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 69, 72–80 (2008)
H.D. Mason, R. Margara, R.M. Winston, R.W. Beard, M.J. Reed, S. Franks, Inhibition of oestradiol production by epidermal growth factor in human granulosa cells of normal and polycystic ovaries. Clin. Endocrinol. (Oxf) 33, 511–517 (1990)
L. Pellatt, L. Hanna, M. Brincat, R. Galea, H. Brain, S. Whitehead, H. Mason, Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J. Clin. Endocrinol. Metab. 92, 240–245 (2007)
D. Monniaux, N. Clemente, J.L. Touze, C. Belville, C. Rico, M. Bontoux, J.Y. Picard, S. Fabre, Intrafollicular steroids and anti-mullerian hormone during normal and cystic ovarian follicular development in the cow. Biol. Reprod. 79, 387–396 (2008)
S.G. Hillier, M. Tetsuka, H.M. Fraser, Location and developmental regulation of androgen receptor in primate ovary. Hum. Reprod. 12, 107–111 (1997)
M.Y. Yang, J.E. Fortune, Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol. Reprod. 75, 924–932 (2006)
A.L. Durlinger, P. Kramer, B. Karels, F.H. de Jong, J.T. Uilenbroek, J.A. Grootegoed, A.P. Themmen, Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140, 5789–5796 (1999)
L.J. Webber, S.A. Stubbs, J. Stark, R.A. Margara, G.H. Trew, S.A. Lavery, K. Hardy, S. Franks, Prolonged survival in culture of preantral follicles from polycystic ovaries. J. Clin. Endocrinol. Metab. 92, 1975–1978 (2007)
D.S. Willis, H. Watson, H.D. Mason, R. Galea, M. Brincat, S. Franks, Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J. Clin. Endocrinol. Metab. 83, 3984–3991 (1998)
H.D. Mason, D.S. Willis, R.W. Beard, R.M. Winston, R. Margara, S. Franks, Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J. Clin. Endocrinol. Metab. 79, 1355–1360 (1994)
P. Chomczynski, N. Sacchi, Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987)
M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Acknowledgments
We would like to thank Dr. Helen Mason and her colleagues at St. Georges University of London, for their instruction regarding follicle dissection and granulosa cell culture techniques. We would also like to thank Mr. Herman Rodriguez at the local abattoir for his help with the extraction of bovine ovaries. This work was supported by grants from: CONICYT 23070163 to partially support the Ph.D. thesis (to NC), FONDECYT: 1071007 (to TS-P), and 1090036 (to HL). NC is recipient of a Ph.D. MD fellowship from the Faculty of Medicine of the University of Chile.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crisosto, N., Sir-Petermann, T., Greiner, M. et al. Testosterone-induced downregulation of anti-Müllerian hormone expression in granulosa cells from small bovine follicles. Endocr 36, 339–345 (2009). https://doi.org/10.1007/s12020-009-9227-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-009-9227-6