Abstract
Periodontitis is a complex and inflammatory disorder characterized by elevated level of tissue-degrading enzymes leading to the destruction of periodontal tissue and ultimately causes loss of the tooth. Apart from standard therapeutic measures used to control periodontal infection and inflammation, matrix metalloproteinase (MMP) control plays an important role in improving outcomes of periodontal therapy. In chronic periodontitis, levels of endogenous tissue inhibitor are not sufficient to inhibit the elevated MMPs, leading to increased pathogenesis of the periodontal disease. Literature evidence revealed adjunctive tissue inhibitors of metalloproteinases (TIMPs) play a pivotal role in managing chronic periodontitis. Recently, a large number of products including collagen, peptidomimetics, non-peptidomimetics, tetracycline, chemically modified tetracycline, and bisphosphonates are being investigated as MMP inhibitors to improve outcomes in periodontal therapy. This review aims to analyze the role of MMPs in periodontitis and summarizes the therapeutic application of TIMPs that could help in selecting an appropriate adjunct to periodontal therapy for better outcomes.
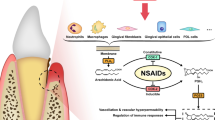
Graphic Abstract




Similar content being viewed by others
References
Honibald EN, Mathew S, Padmanaban J, Sundaram E RR. Perioceutics: matrix metalloproteinase inhibitors as an adjunctive therapy for inflammatory periodontal disease. J Pharm &bioallied Sci. 2012;Suppl 2(S417).
De Souza AP, Da Silva R, Cantazaro-Guimarães S. Matrix metalloproteinases: the most important pathway involved with periodontal destruction. Brazilian J Oral Sci Published online. 2005. https://doi.org/10.20396/bjos.v4i15.8641850.
Yamamoto K, Murphy G, Troeberg L. Extracellular regulation of metalloproteinases. Matrix Biol Published online. 2015. https://doi.org/10.1016/j.matbio.2015.02.007.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. Published online 2014. https://doi.org/10.1038/nrm3904
Hernández-Ríos P, Hernández M, Garrido M, Tervahartiala T, Leppilahti JM, Kuula H, Heikkinen AM, Mäntylä P, Rathnayake N, Nwhator SST. Oral fluid matrix metalloproteinase (MMP)-8 as a diagnostic tool in chronic periodontitis. Met Med. 2011;38(9):817–9.
Parmar NK, Nisha KJ, Guru S PS. Role of matrixmetalloproteinases in periodontal disease—a review. Biomed J Sci Tech Res. 2018;2(1.):2099–2104.
Ozmeric NGC. Chemical inhibition of matrix metalloproteinases for periodontal treatment. Clin Anti-inflamm Anti-Allergy Drugs. 2015;2(1):21–6.
Sorsa T, Tjäderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med Published online. 2006. https://doi.org/10.1080/07853890600800103.
Shin DS, Park JW, Suh JY, Lee JM. The expressions of inflammatory factors and tissue inhibitor of matrix metalloproteinase-2 in human chronic periodontitis with type 2 diabetes mellitus. J Periodontal Implant Sci Published online. 2010. https://doi.org/10.5051/jpis.2010.40.1.33.
Barreiros D, Nelson-Filho P, Paula-Silva FWG, et al. MMP2 and MMP9 are associated with apical periodontitis progression and might be modulated by TLR2 and MyD88. Braz Dent J Published online. 2018. https://doi.org/10.1590/0103-6440201801731.
Carneiro E, Menezes R, Garlet GP, et al. Expression analysis of matrix metalloproteinase-9 in epithelialized and nonepithelialized apical periodontitis lesions. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. Published online 2009. https://doi.org/10.1016/j.tripleo.2008.07.030
Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1β–induced peritonitis. J Immunol Published online. 2013. https://doi.org/10.4049/jimmunol.1202286.
Franco C, Patricia HR, Timo S, Claudia B, Marcela H. Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci Published online. 2017. https://doi.org/10.3390/ijms18020440.
Kawashima N, Suzuki N, Yang G, et al. Kinetics of RANKL, RANK and OPG expressions in experimentally induced rat periapical lesions. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. Published online 2007. https://doi.org/10.1016/j.tripleo.2006.11.036
Menezes R, Garlet TP, Letra A, Bramante CM, Campanelli AP, de CássiaFigueira R, Sogayar MC, Granjeiro JM, Garlet GP. Differential patterns of receptor activator of nuclear factor kappa B ligand/osteoprotegerin expression in human periapical granulomas: possible association with progressive or stable nature of the lesions. J Endod. 2008;34(8):932–8.
Golub LM, Lee HM, Stoner JA, et al. Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol Published online. 2008. https://doi.org/10.1902/jop.2008.070623.
Hwang BM, Chae HS, Jeong YJ, et al. Protein tyrosine phosphatase controls breast cancer invasion through the expression of matrix metalloproteinase-9. BMB Rep Published online. 2013. https://doi.org/10.5483/BMBRep.2013.46.11.053.
Dayton TL, Gocheva V, Miller KM, et al. Isoform-specific deletion of PKM2 constrains tumor initiation in a mouse model of soft tissue sarcoma. Cancer Metab Published online. 2018. https://doi.org/10.1186/s40170-018-0179-2.
Mueller S, Michaelis M, Lindemann S, Gigout A. Effects of three potential anabolic disease-modifying osteoarthritis drugs – sprifermin, IGF1 and BMP7 – on matrix production and the phenotype of articular chondrocytes. Osteoarthr Cartil Published online. 2019. https://doi.org/10.1016/j.joca.2019.02.227.
Staines KA, Prideaux M, Hohenstein P, Buttle DJ, Pitsillides AA, Farquharson C. E11 protein stabilisation by proteasome inhibition promotes osteocyte differentiation and may protect against osteoarthritis bone pathology. Osteoarthr Cartil. 2015;23:A57.
Michigami T. Regulatory mechanisms for the development of growth plate cartilage. Cell Mol Life Sci Published online. 2013. https://doi.org/10.1007/s00018-013-1346-9.
Ogura Y, Tajrishi MM, Sato S, Hindi SM, Kumar A. Therapeutic potential of matrix metalloproteinases in Duchenne muscular dystrophy. Front Cell Dev Biol Published online. 2014. https://doi.org/10.3389/fcell.2014.00011.
Kiili M, Cox SW, Chen HW, et al. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J Clin Periodontol Published online. 2002. https://doi.org/10.1034/j.1600-051x.2002.290308.x.
Kudo Y, Iizuka S, Yoshida M, et al. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J Biol Chem Published online. 2012. https://doi.org/10.1074/jbc.M112.373159.
Baeza M, Garrido M, Hernández-Ríos P, et al. Diagnostic accuracy for apical and chronic periodontitis biomarkers in gingival crevicular fluid: an exploratory study. J Clin Periodontol Published online. 2016. https://doi.org/10.1111/jcpe.12479.
Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci Published online. 2006. https://doi.org/10.2741/1817.
Brighton CT, Wang W, Clark CC. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J Bone Jt Surg - Ser A. Published online 2008. https://doi.org/10.2106/JBJS.F.01437
Murphy G. Riding the metalloproteinase roller coaster. J Biol Chem Published online. 2017. https://doi.org/10.1074/jbc.X117.785295.
Sayed G, Omar G, Ghanem A, Mohamed N, El-Kholy M. Association of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 levels with diabetic foot ulcer in Egyptians with type 2 diabetes mellitus. J Med Sci Res Published online. 2018. https://doi.org/10.4103/jmisr.jmisr_80_18.
Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta - Mol Cell Res. Published online 2010. https://doi.org/10.1016/j.bbamcr.2009.08.006
Leco KJ, Martin EL, Barcroft LC, Gill SE. Matrix metalloproteinase inhibitors. In: Encyclopedia of respiratory medicine, four-volume set. 2006. https://doi.org/10.1016/B0-12-370879-6/00243-X
Egawa N, Koshikawa N, Tomari T, Nabeshima K, Isobe T, Seiki M. Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J Biol Chem Published online. 2006. https://doi.org/10.1074/jbc.M606993200.
Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst Published online. 2008. https://doi.org/10.1093/jnci/djm268.
Cheng G, Fan X, Hao M, Wang J, Zhou X, Sun X. Higher levels of TIMP-1 expression are associated with a poor prognosis in triplenegative breast cancer. Mol Cancer Published online. 2016. https://doi.org/10.1186/s12943-016-0515-5.
Ritter LM, Garfield SH, Thorgeirsson UP. Tissue inhibitor of metalloproteinases-1 (TIMP-1) binds to the cell surface and translocates to the nucleus of human MCF-7 breast carcinoma cells. Biochem Biophys Res Commun Published online. 1999. https://doi.org/10.1006/bbrc.1999.0408.
Johanson M, Zhao XR, Huynh-Ba G, Villar CC. Matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and inflammation in cyclosporine A–induced gingival enlargement: a pilot in vitro study using a three-dimensional model of the human oral mucosa. J Periodontol Published online. 2013. https://doi.org/10.1902/jop.2012.120224.
Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol Published online. 2011. https://doi.org/10.1186/gb-2011-12-11-233.
Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta - Mol Cell Res. Published online 2010. https://doi.org/10.1016/j.bbamcr.2010.01.003
Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood Published online. 2007. https://doi.org/10.1182/blood-2006-10-051060.
Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev Published online. 2008. https://doi.org/10.1007/s10555-007-9105-8.
Peng X, Chen Z, Farshidfar F, et al. Molecular characterization and clinical relevance of metabolic expression subtypes in human cancers. Cell Rep Published online. 2018. https://doi.org/10.1016/j.celrep.2018.03.077.
Higashi S, Hirose T, Takeuchi T, Miyazaki K. Molecular design of a highly selective and strong protein inhibitor against matrix metalloproteinase-2 (MMP-2). J Biol Chem Published online. 2013. https://doi.org/10.1074/jbc.M112.441758.
R. R, S. F, M. H, B. H. Cannabinoids inhibit angiogenic properties of endothelial cells via increasing TIMP-1 release from lung cancer cells. Naunyn Schmiedebergs Arch Pharmacol. Published online 2014.
D’Alessio S, Ferrari G, Cinnante K, et al. Tissue inhibitor of metalloproteinases-2 binding to membrane-type 1 matrix metalloproteinase induces MAPK activation and cell growth by a non-proteolytic mechanism. J Biol Chem Published online. 2008. https://doi.org/10.1074/jbc.M705492200.
Wu ZS, Wu Q, Yang JH, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer Published online. 2008. https://doi.org/10.1002/ijc.23337.
Li Z, Clarke MP, Barker MD, McKie N. TIMP3 mutation in Sorsby’s fundus dystrophy: molecular insights. Expert Rev Mol Med Published online. 2005. https://doi.org/10.1017/S1462399405010045.
Warwick A, Gibson J, Sood R, Lotery A. A rare penetrant TIMP3 mutation confers relatively late onset choroidal neovascularisation which can mimic age-related macular degeneration. Eye Published online. 2016. https://doi.org/10.1038/eye.2015.204.
Vargova V, Pytliak M, Mechirova V. Matrix metalloproteinases and their tissue inhibitors in diabetes, atherosclerosis and prediction of the cardiovascular risk. Curr Enzym Inhib Published online. 2011. https://doi.org/10.2174/157340810794578524.
E Sharma,A Lakhani ST and SK. Role of MMPs in connective tissue breakdown and periodontal disease: a review. Austin Dent Sci. 2019;4(1).
Ingman T, Tervahartiala T, Ding Y, Tschesche H, Haerian A, Kinane DF, Konttinen YTST. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23(12):1127–32.
Tüter G, Kurtiş BSM. Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1. J Periodontol. 2002;73(5):487–93.
Gursoy UK, Könönen E, Pradhan-Palikhe P, et al. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol Published online. 2010. https://doi.org/10.1111/j.1600-051X.2010.01563.x.
Sorsa T, Mäntylä P, Tervahartiala T, Pussinen PJ, Gamonal J, Hernandez M. MMP activation in diagnostics of periodontitis and systemic inflammation. J Clin Periodontol Published online. 2011. https://doi.org/10.1111/j.1600-051X.2011.01753.x.
Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol Published online. 2013. https://doi.org/10.1038/nri3499.
Amǎlinei C, Cǎruntu ID, Giu̧cǎ SE, Bǎlan RA. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. Published online. 2010.
Torok S, Rezeli M, Kelemen O, et al. Limited tumor tissue drug penetration contributes to primary resistance against angiogenesis inhibitors. Theranostics Published online. 2017. https://doi.org/10.7150/thno.16767.
Kasaoka T, Nishiyama H, Okada M, Nakajima M. Matrix metalloproteinase inhibitor, MMI270 (CGS27023A) inhibited hematogenic metastasis of B16 melanoma cells in both experimental and spontaneous metastasis models. Clin Exp Metastasis Published online. 2008. https://doi.org/10.1007/s10585-008-9198-7.
Fisher JF, Mobashery S. Recent advances in MMP inhibitor design. Cancer Metastasis Rev Published online. 2006. https://doi.org/10.1007/s10555-006-7894-9.
Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov Published online. 2014. https://doi.org/10.1038/nrd4390.
Maquoi E, Sounni NE, Devy L, et al. Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin Cancer Res Published online. 2004. https://doi.org/10.1158/1078-0432.CCR-04-0125.
Farina AR, Mackay AR. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers (Basel). Published online. 2014. https://doi.org/10.3390/cancers6010240
Gonçalves PF, Huang H, McAninley S, et al. Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J Periodontol Published online. 2013 Dec;84(12):1801–8. https://doi.org/10.1902/jop.2013.130002.
Gu Y, Lee HM, Sorsa T, et al. Non-antibacterial tetracyclines modulate mediators of periodontitis and atherosclerotic cardiovascular disease: a mechanistic link between local and systemic inflammation. Pharmacol Res Published online. 2011. https://doi.org/10.1016/j.phrs.2011.06.023.
Needleman I, Suvan J, Gilthorpe MS, et al. A randomized-controlled trial of low-dose doxycycline for periodontitis in smokers. J Clin Periodontol Published online. 2007. https://doi.org/10.1111/j.1600-051X.2007.01058.x.
Caton J, Ryan ME. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol Res Published online. 2011. https://doi.org/10.1016/j.phrs.2010.12.003.
Acharya MR, Venitz J, Figg WD, Sparreboom A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist Updat Published online. 2004. https://doi.org/10.1016/j.drup.2004.04.002.
Ahler E, Sullivan WJ, Cass A, et al. Doxycycline alters metabolism and proliferation of human cell lines. PLoS One Published online. 2013. https://doi.org/10.1371/journal.pone.0064561.
Farhad SZ, Aminzadeh A, Mafi M, Barekatain M, Naghney M, Ghafari MR. The effect of adjunctive low-dose doxycycline and licorice therapy on gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. Dent Res J (Isfahan). Published online. 2013.
Kurdukar PA, Kurdukar AA, Mahale SA, Amol D, Beldar M. Biomarkers in gingival crevicular fluid. IOSR J Dent Med Sci Ver IX: Published online. 2015.
Antonio RC, Ceron CS, Rizzi E, Coelho EB, Tanus-Santos JE, Gerlach RF. Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Mol Cell Biochem Published online. 2014. https://doi.org/10.1007/s11010-013-1848-7.
Emingil G, Atilla G, Sorsa T, Luoto H, Kirilmaz L, Baylas H. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 Levels in chronic periodontitis. J Periodontol Published online. 2004. https://doi.org/10.1902/jop.2004.75.1.106.
Wang X, Rojas-Quintero J, Wilder J, Tesfaigzi Y, Zhang D, Owen CA. Tissue inhibitor of metalloproteinase-1 promotes polymorphonuclear neutrophil (PMN) pericellular proteolysis by anchoring matrix metalloproteinase-8 and -9 to PMN surfaces. J Immunol Published online. 2019. https://doi.org/10.4049/jimmunol.1801466.
Golub LM, McNamara TF, Ryan ME, et al. Adjunctive treatment with subantimicrobial doses of doxycycline: effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol Published online. 2001. https://doi.org/10.1034/j.1600-051x.2001.028002146.x.
Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. In: Annals of periodontology / the American Academy of Periodontology. 2003. https://doi.org/10.1902/annals.2003.8.1.12
Golub LM, Lee HM. Periodontal therapeutics: current host-modulation agents and future directions. Periodontol 2000. Published online. 2020. https://doi.org/10.1111/prd.12315
Swamy D, Sanivarapu S, Moogla S, Kapalavai V. Chemically modified tetracyclines: the novel host modulating agents. J Indian Soc Periodontol Published online. 2015. https://doi.org/10.4103/0972-124X.149934.
Syed S, Takimoto C, Hidalgo M, et al. A phase I and pharmacokinetic study of Col-3 (Metastat), an oral tetracycline derivative with potent matrix metalloproteinase and antitumor properties. Clin Cancer Res Published online. 2004. https://doi.org/10.1158/1078-0432.CCR-04-0804.
Salo T, Soini Y, Oiva J, et al. Chemically modified tetracyclines (CMT-3 and CMT-8) enable control of the pathologic remodellation of human aortic valve stenosis via MMP-9 and VEGF inhibition. Int J Cardiol Published online. 2006. https://doi.org/10.1016/j.ijcard.2005.07.042.
Hidalgo M, Eckhardt SG. Matrix metalloproteinase inhibitors: how can we optimize their development? Ann Oncol Published online. 2001. https://doi.org/10.1023/A:1011198530099.
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. Published online. 2016. https://doi.org/10.3109/14756366.2016.1161620
Bildt MM, Bloemen M, Kuijpers-Jagtman AM, Von Den Hoff JW. Matrix metalloproteinase inhibitors reduce collagen gel contraction and -smooth muscle actin expression by periodontal ligament cells. J Periodontal Res Published online. 2009. https://doi.org/10.1111/j.1600-0765.2008.01127.x.
Nakaya H, Osawa G, Iwasaki N, Cochran DL, Kamoi K, Oates TW. Effects of bisphosphonate on matrix metalloproteinase enzymes in human periodontal ligament cells. J Periodontol Published online. 2000. https://doi.org/10.1902/jop.2000.71.7.1158.
Visscher DW, Höyhtyä M, Ottosen SK, et al. Enhanced expression of tissue inhibitor of metalloproteinase-2 (TIMP-2) in the stroma of breast carcinomas correlates with tumor recurrence. Int J Cancer Published online. 1994. https://doi.org/10.1002/ijc.2910590308.
Kwak TW, Park SB, Kim HJ, Jeong YIL, Kang DH. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. Onco Targets Ther Published online. 2017. https://doi.org/10.2147/OTT.S112364.
Scoditti E, Calabriso N, Massaro M, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. In: Archives of Biochemistry and Biophysics. 2012. https://doi.org/10.1016/j.abb.2012.05.003
Sartor L, Pezzato E, Dell’aica I, Caniato R, Biggin S, Garbisa S. Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol. Published online. 2002. https://doi.org/10.1016/S0006-2952(02)01069-9
Moroy G, Bourguet E, Decarme M, et al. Inhibition of human leukocyte elastase, plasmin and matrix metalloproteinases by oleic acid and oleoyl-galardin derivative(s). Biochem Pharmacol Published online. 2011. https://doi.org/10.1016/j.bcp.2010.12.001.
Huet E, Cauchard JH, Berton A, et al. Inhibition of plasmin-mediated prostromelysin-1 activation by interaction of long chain unsaturated fatty acids with kringle 5. Biochem Pharmacol Published online. 2004. https://doi.org/10.1016/j.bcp.2003.09.033.
Gaultier F, Foucault-Bertaud A, Lamy E, et al. Effects of a vegetable extract from Lupinus albus (LU105) on the production of matrix metalloproteinases (MMP1, MMP2, MMP9) and tissue inhibitor of metalloproteinases (TIMP1, TIMP2) by human gingival fibroblasts in culture. Clin Oral Investig Published online. 2003. https://doi.org/10.1007/s00784-003-0210-y.
Gaultier F, Ejeil A-L, Dridi S-M, et al. Lupinus albus, a novel vegetable extract with metalloproteinase inhibitory properties: a potential periodontal therapy. J Periodontol Published online. 2005. https://doi.org/10.1902/jop.2005.76.8.1329.
Song SE, Choi BK, Kim SN, et al. Inhibitory effect of procyanidin oligomer from elm cortex on the matrix metalloproteinases and proteases of periodontopathogens. J Periodontal Res Published online. 2003. https://doi.org/10.1034/j.1600-0765.2003.02604.x.
Maske TT, Kuper NK, Cenci MS, Huysmans MCDNJM. Chlorhexidine, a matrix metalloproteinase inhibitor and the development of secondary caries wall lesions in a microcosm biofilm model. Caries Res. Published online. 2019. https://doi.org/10.1159/000490195
Javaid M, Bi J, Biddle C, Tsai CM, Häkkinen L, Kim H. Platelet factor 4 upregulates matrix metalloproteinase‐1 production in gingival fibroblasts. J Periodontal Res. 2017 Aug;52(4):787–92.
Boşca AB, Miclăuş V, Raţiu C, Melincovici C. Matrix metalloproteinase-8—A Salivary diagnostic biomarker related to soft tissue destruction in chronic periodontitis. Ann RSCB. 2012 Jun 1;17:251–7.
Rai B, Kharb S, Jain R, Anand SC. Biomarkers of periodontitis in oral fluids. J Oral Sci. 2008;50(1):53–6.
Smith PC, Muñoz VC, Collados L, Oyarzún AD. In situ detection of matrix metalloproteinase‐9 (MMP‐9) in gingival epithelium in human periodontal disease. Journal of Periodontal research. 2004 Apr;39(2):87-92.
Itagaki M, Kubota T, Tai H, Shimada Y, Morozumi T, Yamazaki K. Matrix metalloproteinase‐1 and‐3 gene promoter polymorphisms in Japanese patients with periodontitis. J Clin Periodontol. 2004 Sep;31(9):764–9
Azmak N, Atilla G, Luoto H, Sorsa T. The Effect of Subgingival Controlled‐Release Delivery of Chlorhexidine Chip onClinical Parameters and Matrix Metalloproteinase‐8 Levels in Gingival Crevicular Fluid. J Periodontol. 2002 Jun;73(6):608–15.
Yek EC, Cintan S, Topcuoglu N, Kulekci G, Issever H, Kantarci A. Efficacy of amoxicillin and metronidazole combination for the management of generalized aggressive periodontitis. J Periodontol. 2010 Jul;81(7):964–74.
Yuan C, Liu X, Zheng S. Matrix metalloproteinase-8 levels in oral samples as a biomarker for periodontitis in the Chinese population: an observational study. BMC oral health. 2018 Dec;18(1):1–6.
Gursoy UK, Könönen E, Huumonen S, Tervahartiala T, Pussinen PJ, Suominen AL, Sorsa T. Salivary type I collagen degradation end‐products and related matrix metalloproteinases in periodontitis. J Clin Periodontol. 2013 Jan;40(1):18–25.
Hardy DC, Ross JH, Schuyler CA, Leite RS, Slate EH, Huang Y. Matrix metalloproteinase‐8 expression in periodontal tissues surgically removed from diabetic and non‐diabetic patients with periodontal disease. J Clin Periodontol. 2012 Mar;39(3):249–55.
Yakob M, Kari K, Tervahartiala T, Sorsa T, Söder PÖ, Meurman JH, Söder B. Associations of periodontal microorganisms with salivary proteins and MMP‐8 in gingival crevicular fluid. J Clin Periodontol. 2012 Mar;39(3):256–63.
Leppilahti JM, Ahonen MM, Hernández M, Munjal S, Netuschil L, Uitto VJ, Sorsa T, Mäntylä P. Oral rinse MMP‐8 point‐of‐care immuno test identifies patients with strong periodontal inflammatory burden. Oral diseases. 2011 Jan;17(1):115–22.
Kim JB, Jung MH, Cho JY, Park JW, Suh JY, Lee JM. The influence of type 2 diabetes mellitus on the expression of inflammatory mediators and tissue inhibitor of metalloproteinases-2 in human chronic periodontitis. Journal of periodontal & implant science. 2011 Jun 1;41(3):109–16.
Özçaka Ö, Bıçakcı N, Pussinen P, Sorsa T, Köse T, Buduneli N. Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis. Oral Dis. 2011 Jan;17(1):68–76.
Marcaccini AM, Meschiari CA, Zuardi LR, De Sousa TS, Taba Jr M, Teofilo JM, Jacob‐Ferreira AL, Tanus‐Santos JE, Novaes Jr AB, Gerlach RF. Gingival crevicular fluid levels of MMP‐8, MMP‐9, TIMP‐2, and MPO decrease after periodontal therapy. J Clin Periodontol.2010 Feb;37(2):180–90.
Gursoy UK, Könönen E, Pradhan‐Palikhe P, Tervahartiala T, Pussinen PJ, Suominen‐Taipale L, Sorsa T. Salivary MMP‐8, TIMP‐1, and ICTP as markers of advanced periodontitis. J Clin Periodontol. 2010 Jun;37(6):487–93.
Hernández M, Gamonal J, Tervahartiala T, Mäntylä P, Rivera O, Dezerega A, Dutzan N, Sorsa T. Associations between matrix metalloproteinase‐8 and‐14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: A longitudinal study. J Periodontol. 2010 Nov;81(11):1644–52.
Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes Jr AB, Taba Jr M. Salivary interleukin‐6, matrix metalloproteinase‐8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol. 2010 Mar;81(3):384–91.
Oyarzún A, Arancibia R, Hidalgo R, Peñafiel C, Cáceres M, González MJ, Martínez J, Smith PC. Involvement of MT1‐MMP and TIMP‐2 in human periodontal disease. Oral Dis. 2010 May;16(4):388–95.
Marcaccini AM, Novaes Jr AB, Meschiari CA, Souza SL, Palioto DB, Sorgi CA, Faccioli LH, Tanus-Santos JE, Gerlach RF. Circulating matrix metalloproteinase-8 (MMP-8) and MMP-9 are increased in chronic periodontal disease and decrease after non-surgical periodontal therapy. Clinica chimica acta. 2009 Nov 3;409(1-2):117–22.
Maeso G, Bravo M, Bascones A. Levels of metalloproteinase-2 and-g and tissue inhibitor of matrix metalloproteinase-1 in gingival crevicular fluid of patients with periodontitis, gingivitis, and healthy gingiva. Quintessence International. 2007 Mar 1;38(3).
Hernández Ríos M, Sorsa T, Obregón F, Tervahartiala T, Valenzuela MA, Pozo P, Dutzan N, Lesaffre E, Molas M, Gamonal J. Proteolytic roles of matrix metalloproteinase (MMP)‐13 during progression of chronic periodontitis: initial evidence for MMP‐13/MMP‐9 activation cascade. J Clin Periodontol. 2009 Dec;36(12):1011–7.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• MMPs are the key mediators of tissue destruction in periodontitis
• MMPs can be inhibited by TIMPs but they have short half-lives and non-selective
• Doxycycline can inhibit the activity of MMPs in periodontitis with beneficial clinical outcome
• MMPs and TIMPs levels in saliva could be diagnostically utilized as a biomarker for the early detection of periodontitis
Rights and permissions
About this article
Cite this article
Sahu, A., Bhuyan, S.K., Bhuyan, R. et al. Understanding the Role of Metalloproteinases and Their Inhibitors in Periodontology. Clinic Rev Bone Miner Metab 19, 36–49 (2021). https://doi.org/10.1007/s12018-021-09281-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-021-09281-y