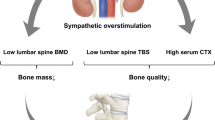
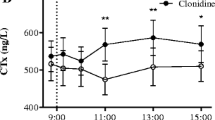
Abstract
Known bone functions include maintaining the homeostasis of the calcium–phosphate metabolism, repairing damage produced by daily exercise and maintaining the bone architecture according to mechanical requirements, meaning that bone remodeling is a true homeostatic function. Bone is a dynamic tissue that is constantly changing through bone remodeling, which requires a lot of energy. The sympathetic nervous system contributes to bone remodeling and is one form of interaction between the skeleton and the brain, through leptin, an adipocyte-derived hormone, which uses this route to induce expression of the gene for the receptor activator of nuclear factor kappa-β ligand, an osteoclast differentiation factor. This review summarizes basic research findings on the role of the sympathetic nervous system in bone metabolism.

Similar content being viewed by others
References
Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–8.
Rappaport R. Reciprocal regulation of bone and energy metabolism. GGH. 2009;25:24–5.
Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–801.
Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14.
Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49.
Harada SI, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–55.
La Gros M. Disposition des nerfs des os. Bull Soc Anat Paris. 1846;21:369–72.
Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–9.
Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M. Neuropeptide Y, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988;25:119–25.
Bjurholmb A, Kreicbergs A, Brodin E, Schultzberg M. Substance P and CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165–71.
Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of rats. Cell Tissue Res. 1991;264:469–80.
Castañeda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR, Mantyh PW. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196–207.
García-Castellano JM, Díaz-Herrera P, Morcuende JA. Is bone a target-tissue for the nervous system? New advances on the understanding of their interactions. Iowa Orthop J. 2000;20:49–58.
Denes A, Boldogkoi Z, Uhereczky G, Hornyak A, Rusvai M, Palkovits M, Kovacs KJ. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005;134:947–63.
Francis GS. Modulation of peripheral sympathetic nerve transmission. J Am Coll Cardiol. 1988;12:250–4.
Maassen AP. The influence of adrenalectomy on the growth of rats. Arch Int Pharmacodyn Ther. 1952;88:473–81.
Lipski S. Effects of beta-adrenergic stimulation on bone marrow function in normal and sublethally irradiated mice. The effect of isoprotenerol on cAMP content in bone marrow cells in vivo and in vitro. Int J Radiat Biol Relat Phys Chem Med. 1976;29:359–66.
Moore RE, Smith CK 2nd, Bailey CS, Voelkel EF, Tashjian AH Jr. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993;23:301–15.
Kellenberger S, Muller K, Richener H, Bilbe G. Formoterol and isoproterenol induce c- fos gene expression in osteoblast- like cells by activating beta2-adrenergic receptors. Bone. 1998;22:471–8.
Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett. 1997;233:125–8.
Togari A. Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech. 2002;58:77–84.
Huang HH, Brennan TC, Muir MM, Mason RS. Functional alpha1-and beta2- adrenergic receptors in human osteoblasts. Cell Physiol. 2009;220:267–75.
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17.
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20.
Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–63.
Ligget SB. Update on current concepts of the molecular basis of B2-adrenergic-receptor signaling. J Allergy Clin Immunol. 2002;110:S223–7.
Benovic JL. Novel beta2-adrenergic receptor signaling pathways. J Allergy Clin Immunol. 2002;110(6 Suppl):S229–35.
Lin FT, Daaka Y, Lefkowitz RJ. Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulinlike growth factor I receptor. J Biol Chem. 1998;273:31640–3.
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry Syndrome. Cell. 2004;117:387–98.
Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76.
Takeuchi T, Tsuboi T, Arai M, Togari A. Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem Pharmacol. 2001;61:579–86.
Pierroz D, Bouxsein M, Rizzoli R, Ferrari S. Combined treatment with a b-blocker and intermittent PTH improves bone mass and microarchitecture in ovariectomized mice. Bone. 2006;39:260–7.
Frediani U, Becherini L, Lasagni L, Tanini A, Brandi ML. Catecholamines modulate growth and differentiation of human preosteoclastic cells. Osteoporos Int. 1996;6:14–21.
Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–9.
Li H, Fong C, Chen Y, Cai G, Yang M. Beta2- and beta3-, but not beta1-adrenergic receptors are involved in osteogenesis of mouse mesenchymal stem cells via cAMP/PKA signaling. Arch Biochem Biophys. 2010;496:77–83.
Owen TA, Bortell R, Yocum SA, Smock SL, Zhang M, Abate C, Shalhoub V, Aronin N, Wright KL, van Wijnen AJ. Coordinate occupancy of AP-1 sites in the vitamin D-responsive and CCAAT box elements by Fos-Jun in the osteocalcin gene: model for phenotype suppression of transcription. Proc Natl Acad Sci USA. 1990;87:9990–4.
Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetología. 2011;54:1291–7.
Riggs BL, Melton LJ 3rd. Involutional osteoporosis. N Engl J Med. 1986;314:1676–86.
Zhao LJ, Liu YJ, Yuan Liu P, Hamilton J, Recker RR, Den HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–6.
Ricci TA, Heymsfield SB, Pierson RN Jr, Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73:347–52.
Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–4.
Tremollieres FA, Pouilles JM, Ribot C. Vertebral postmenopausal bone loss is reduced in overweight women: a longitudinal study in 155 early postmenopausal women. J Clin Endocrinol Metab. 1993;77:683–6.
Zhang F, Cehn Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam Horm. 2005;71:345–72.
Auwerx J, Staels B. Leptin. Lancet. 1998;351:737–42.
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207.
Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG Jr. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–60.
Yadav VK, Karsenty G. Leptin dependent co-regulation of bone and energy metabolism. Aging (Albany NY). 2009;1:954–6.
Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6.
Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G. Cart overexpression is the only identificable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology. 2006;147:3196–202.
Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin regulated bone formation. Cell. 2005;122:803–15.
Rosen CJ. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab. 2008;7:7–10.
Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya K, Takeda S. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13:1234–40.
Cirmanová V, Bayer M, Starka L, Zajickova K. The effect of leptin on bone: an evolving concept of action. Physiol Res. 2008;57:S143–51.
Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43.
Allison SJ, Baldock P, Sainsbury A, Enriquez R, Lee NJ, Lin EJ, Klugmann M, During M, Eisman JA, Li M, Pan LC, Herzog H, Gardiner EM. Conditional deletion of hypothalamic Y2 receptors reverts gonadectomy induced bone loss in adult mice. J Biol Chem. 2006;281:23436–44.
Sato N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48:1787–93.
Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency introduces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–83.
Reid IR. Leptin deficiency-lessons in regional differences in the regulation of bone mass. Bone. 2004;34:369–71.
Young JB, Landsberg L. Catecholamines and the adrenal medulla. In: Wilson JD, Foster DW, Kroenberg HM, Larsen PR, editors. Williams textbook of endocrinology. 9th ed. Philadelphia: W.B. Saunders; 1998. p. 665–728.
Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, Guo XE, Karsenty G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208:841–51.
Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V, Pichon C, Courteix D. Low dose beta-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J Cell Physiol. 2008;217:819–27.
Pierroz DD, Bouxsein ML, Muzzin P, Rizzoli R, Ferrari SL. Bone loss following ovariectomy is maintained in absence of adrenergic receptor beta1 and beta2 signaling. J Bone Miner Res. 2005;20(Suppl. 1):S277.
Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, Ferrari SL. Mice lacking beta-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology. 2009;150:144–52.
Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylylcyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–58.
Kondo H, Nifuji A, Takeda S, Ezura Y, Rittling SR, Denhardt DT, Nakashima K, Karsenty G, Noda M. Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem. 2005;280:30192–200.
Zhang W, Kanehara M, Zhang Y, Wang X, Ishida T. Blocker and other analogous treatments that affect bone mass and sympathetic nerve activity inovariectomized rats. Am J Chin Med. 2007;35:89–101.
Levasseur R, Sabatier JP, Potrel-Burgot C, Lecoq B, Creveuil C, Marcelli C. Sympathetic nervous system as transmitter of mechanical loading in bone. Joint Bone Spine. 2003;70:515–9.
Rodrigues WF, Madeira MF, da Silva TA, Clemente-Napimoga JT, Miguel CB, Dias-da-Silva VJ, Barbosa-Neto O, Lopes AH, Napimoga MH. Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. Br J Pharmacol. 2012;165:2140–51.
Minkowitz B, Boskey AL, Lane JM, Pearlman HS, Vigorita VJ. Effects of propranolol on bone metabolism in the rat. J Orthop Res. 1991;9:869–75.
Baek K, Hwang HR, Park HJ, Kwon A, Qadir AS, Baek JH. Propranolol, a β-adrenergic antagonist, attenuates the decrease in trabecular bone mass in high calorie diet fed growing mice. BMB Rep. 2014;47:506–11.
Elefteriou F, Campbell P, Ma Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int. 2014;94:140–51.
Pataki A, Muller K, Bilbe G, Green JR, Glatt M. Anabolic effects of beta2-agonists, formoterol and salbutamol on cancellous bone ovariectomized (ovx) rat. Bone. 1996;9:A116.
Arai M, Sato T, Takeuchi S, Goto S, Togari A. Dose effects of butoxamine, a selective β2-adrenoceptor antagonist, on bone metabolism in spontaneously hypertensive rat. Eur J Pharmacol. 2013;701:7–13.
Bonnet N, Brunet-Imbault B, Arlettaz A, Horcajada MN, Collomp K, Benhamou CL, Courteix D. Alteration of trabecular bone under chronic beta2 agonists treatment. Med Sci Sports Exerc. 2005;37:1493–501.
de Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res. 2005;20:2159–68.
Marenzana M, De Souza RL, Chenu C. Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone. 2007;41:206–15.
Fonseca TL, Jorgetti V, Costa CC, Capelo LP, Covarrubias AE, Moulatlet AC, Teixeira MB, Hesse E, Morethson P, Beber EH, Freitas FR, Wang CC, Nonaka KO, Oliveira R, Casarini DE, Zorn TM, Brum PC, Gouveia CH. Double disruption of alpha2A- and alpha2C-adrenoceptors results in sympathetic hyperactivity and high-bone-mass phenotype. J Bone Miner Res. 2011;26:591–603.
Choi YK, Lee JY, Lee SJ, Chung CP, Park YJ. Alpha-adrenergic blocker mediated osteoblastic stem cell differentiation. Biochem Biophys Res Commun. 2011;16(416):232–8.
Disclosures
Conflict of interest
The authors (Marta Gonzalez-Rozas, Antonio Dueñas-Laita and José-Luis Pérez-Castrillón) do not have a potential conflict of interest directly or indirectly related to the research.
Animal/Human Studies
This article does not include any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalez-Rozas, M., Dueñas-Laita, A. & Perez-Castrillon, J.L. The β-Adrenergic System and Bone Mineral Remodeling. Clinic Rev Bone Miner Metab 13, 114–124 (2015). https://doi.org/10.1007/s12018-015-9183-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-015-9183-z