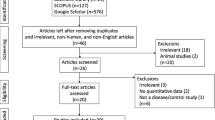
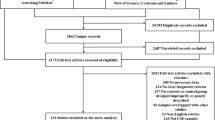
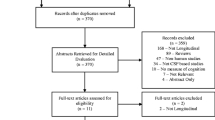
Abstract
The soluble amyloid protein procurer α (sAPPα) and β (sAPPβ) have been postulated as promising new cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease (AD) and multiple other neurodegenerative diseases, but have failed to meet expectations with their often discordant and even contradictory findings to date. The aim of the study was to systematically explore this issue. Cochrane Library, PubMed, and CNKI were systematically searched without language or date restrictions. This network meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and also adhered to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. Twenty studies, comprising ten groups, were eligible and included. Overall, 19 eligible studies with 1634 patients contributed to the analysis of CSF sAPPα levels and 16 eligible studies with 1684 patients contributed to the analysis of CSF sAPPβ levels. CSF sAPPβ levels are significantly higher in AD than in corticobasal syndrome (CBS) and progressive supranuclear palsy (PSP); higher in Control than in Depression, CBS and PSP; higher in Parkinson’s disease dementia (PDD) than in CBS and PSP; higher in mild cognitive impairment progressed to AD dementia during the follow-up period (pMCI) than in Depression and PSP; higher in stable mild cognitive impairment (sMCI) than in Depression. With regard to CSF sAPPα levels, there were no significant difference among groups. However, surprisingly, the resultant rankings graphically showed that pMCI populations have the highest levels of CSF sAPPα and sAPPβ. Furthermore, it seemed there was a positive correlation between CSF sAPPα and sAPPβ levels. The measurement of CSF sAPPα and sAPPβ levels may provide an alternative method for the diagnosis of early-stage AD, pMCI, which is conducive to preventive therapy.




Similar content being viewed by others
Data Availability
All data of the articles included in our research had been published online and are available to investigators.
Abbreviations
- AD:
-
Alzheimer’s disease
- CBS:
-
Corticobasal syndrome
- CSF:
-
Cerebrospinal fluid
- DLB:
-
Dementia with Lewy bodies
- FTD:
-
Frontotemporal dementia
- NMA:
-
Network meta-analysis
- PDD:
-
Parkinson’s disease dementia
- pMCI:
-
Mild cognitive impairment progressed to AD dementia during the follow-up period
- PSP:
-
Progressive supranuclear palsy
- sAPPα:
-
Soluble amyloid protein procurer α
- sAPPβ:
-
Soluble amyloid protein procurer β
- sMCI:
-
Stable mild cognitive impairment
- VaD:
-
Vascular dementia
References
Alcolea, D., Vilaplana, E., Suárez-Calvet, M., Illán-Gala, I., Blesa, R., Clarimón, J., et al. (2017). CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology,89(2), 178–188.
Alexopoulos, P., Tsolakidou, A., Roselli, F., Arnold, A., Grimmer, T., Westerteicher, C., et al. (2012). Clinical and neurobiological correlates of soluble amyloid precursor proteins in the cerebrospinal fluid. Alzheimer’s and Dementia,8(4), 304–311.
Araki, W., Hattori, K., Kanemaru, K., Yokoi, Y., Omachi, Y., Takano, H., et al. (2017). Re-evaluation of soluble APP-α and APP-β in cerebrospinal fluid as potential biomarkers for early diagnosis of dementia disorders. Biomarker Research,5, 28.
Cheng, X., He, P., Lee, T., Yao, H., Li, R., & Shen, Y. (2014). High activities of BACE1 in brains with mild cognitive impairment. The American Journal of Pathology,184(1), 141–147.
Chow, V. W., Mattson, M. P., Wong, P. C., & Gleichmann, M. (2010). An overview of APP processing enzymes and products. NeuroMolecular Medicine,12(1), 1–12.
Cuchillo-Ibañez, I., Lopez-Font, I., Boix-Amorós, A., Brinkmalm, G., Blennow, K., Molinuevo, J. L., et al. (2015). Heteromers of amyloid precursor protein in cerebrospinal fluid. Molecular Neurodegeneration,10, 2.
Deng, J., Habib, A., Obregon, D. F., Barger, S. W., Giunta, B., Wang, Y. J., et al. (2015). Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3β signaling pathway. Journal of Neurochemistry,135(3), 630–637.
de Leon, M. J., DeSanti, S., Zinkowski, R., Mehta, P. D., Pratico, D., Segal, S., et al. (2004). MRI and CSF studies in the early diagnosis of Alzheimer’s disease. Journal of Internal Medicine,256(3), 205–223.
DerSimonian, R., & Laird, N. (2015). Meta-analysis in clinical trials revisited. Contemporary Clinical Trials,45(Pt A), 139–145.
Efthimiopoulos, S., Vassilacopoulou, D., Ripellino, J. A., Tezapsidis, N., & Robakis, N. K. (1996). Cholinergic agonists stimulate secretion of soluble full-length amyloid precursor protein in neuroendocrine cells. Proceedings of the National academy of Sciences of the United States of America,93(15), 8046–8050.
Fellgiebel, A., Kojro, E., Müller, M. J., Scheurich, A., Schmidt, L. G., & Fahrenholz, F. (2009). CSF APPs alpha and phosphorylated tau protein levels in mild cognitive impairment and dementia of Alzheimer’s type. Journal of Geriatric Psychiatry and Neurology,22(1), 3–9.
Furukawa, K., Sopher, B. L., Rydel, R. E., Begley, J. G., Pham, D. G., Martin, G. M., et al. (1996). Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. Journal of Neurochemistry,67(5), 1882–1896.
Gabelle, A., Roche, S., Gény, C., Bennys, K., Labauge, P., Tholance, Y., et al. (2010). Correlations between soluble α/β forms of amyloid precursor protein and Aβ38, 40, and 42 in human cerebrospinal fluid. Brain Research,1357, 175–183.
Gabelle, A., Roche, S., Gény, C., Bennys, K., Labauge, P., Tholance, Y., et al. (2011). Decreased sAβPPβ, Aβ38, and Aβ40 cerebrospinal fluid levels in frontotemporal dementia. Journal of Alzheimer’s Disease,26(3), 553–563.
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet,388(10053), 1545–1602.
GBD 2015 Mortality and Causes of Death Collaborators. (2016). Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet,388(10053), 1459–1544.
GBD 2016 Causes of Death Collaborators. (2017). Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet,390(10100), 1151–1210.
GBD 2016 Dementia Collaborators. (2019). Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology,18(1), 88–106.
Habib, A., Sawmiller, D., & Tan, J. (2017). Restoring soluble amyloid precursor protein α functions as a potential treatment for alzheimer’s disease. Journal of Neuroscience Research,95(4), 973–991.
Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O (2010) Evaluation of CSF biomarkers as predictors of Alzheimer’s disease: A clinical follow-up study of 4.7 years. Journal of Alzheimer’s Disease 21(4):1119–1128
Kantarci, K., Weigand, S. D., Petersen, R. C., Boeve, B. F., Knopman, D. S., Gunter, J., et al. (2007). Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging,28(9), 1330–1339.
Kodamullil, A. T., Zekri, F., Sood, M., Hengerer, B., Canard, L., McHale, D., et al. (2017). Trial watch: Tracing investment in drug development for Alzheimer disease. Nature Reviews Drug Discovery,16(12), 819.
Lannfelt, L., Basun, H., Wahlund, L. O., Rowe, B. A., & Wagner, S. L. (1995). Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer’s disease. Nature Medicine,1(8), 829–832.
Lewczuk, P., Kamrowski-Kruck, H., Peters, O., Heuser, I., Jessen, F., Popp, J., et al. (2010). Soluble amyloid precursor proteins in the cerebrospinal fluid as novel potential biomarkers of Alzheimer’s disease: a multicenter study. Molecular Psychiatry,15(2), 138–145.
Lewczuk, P., Popp, J., Lelental, N., Kölsch, H., Maier, W., Kornhuber, J., et al. (2012). Cerebrospinal fluid soluble amyloid-β protein precursor as a potential novel biomarkers of Alzheimer’s disease. Journal of Alzheimer’s Disease,28(1), 119–125.
Magdalinou, N. K., Paterson, R. W., Schott, J. M., Fox, N. C., Mummery, C., Blennow, K., et al. (2015). A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. Journal of Neurology, Neurosurgery and Psychiatry,86(11), 1240–1247.
Miyajima, M., Nakajima, M., Ogino, I., Miyata, H., Motoi, Y., & Arai, H. (2013). Soluble amyloid precursor protein α in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. European Journal of Neurology,20(2), 236–242.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ,339, b2535.
Moriya, M., Miyajima, M., Nakajima, M., Ogino, I., & Arai, H. (2015). Impact of cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus on the amyloid cascade. PLoS ONE,10(3), e0119973.
Mulugeta, E., Londos, E., Hansson, O., Ballard, C., Skogseth, R., Minthon, L., et al. (2011). Cerebrospinal fluid levels of sAPPα and sAPPβ in lewy body and alzheimer’s disease: Clinical and neurochemical correlates. International Journal of Alzheimer’s Disease,2011, 495025.
Nhan, H. S., Chiang, K., & Koo, E. H. (2015). The multifaceted nature of amyloid precursor protein and its proteolytic fragments: Friends and foes. Acta Neuropathologica,129(1), 1–19.
Nikolaev, A., McLaughlin, T., O’Leary, D. D., & Tessier-Lavigne, M. (2009). APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature,457(7232), 981–989.
O’Brien, R. J., & Wong, P. C. (2011). Amyloid precursor protein processing and Alzheimer’s disease. Annual Review of Neuroscience,34, 185–204.
Olsson, B., Lautner, R., Andreasson, U., Öhrfelt, A., Portelius, E., Bjerke, M., et al. (2016). CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. The Lancet. Neurology,15(7), 673–684.
Perneczky, R., Alexopoulos, P., & Kurz, A. (2014). Soluble amyloid precursor proteins and secretases as Alzheimer’s disease biomarkers. Trends in Molecular Medicine,20(1), 8–15.
Perneczky, R., Tsolakidou, A., Arnold, A., Diehl-Schmid, J., Grimmer, T., Förstl, H., et al. (2011). CSF soluble amyloid precursor proteins in the diagnosis of incipient Alzheimer disease. Neurology,77(1), 35–38.
Peskind, E. R., Leverenz, J., Farlow, M. R., Ito, R. K., Provow, S. A., Siegel, R. S., et al. (1997). Clinicopathologic correlations of soluble amyloid beta-protein precursor in cerebrospinal fluid in patients with Alzheimer disease and controls. Alzheimer Disease and Associated Disorders,11(4), 201–206.
Popp, J., Lewczuk, P., Kölsch, H., Meichsner, S., Maier, W., Kornhuber, J., et al. (2012). Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. Journal of Neurochemistry,123(2), 310–316.
Post, A., Ackl, N., Rücker, M., Schreiber, Y., Binder, E. B., Ising, M., et al. (2006). Toward a reliable distinction between patients with mild cognitive impairment and Alzheimer-type dementia versus major depression. Biological Psychiatry,59(9), 858–862.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. (2000) Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Sastre, M., Dewachter, I., Rossner, S., Bogdanovic, N., Rosen, E., Borghgraef, P., et al. (2006). Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proceedings of the National academy of Sciences of the United States of America,103(2), 443–448.
Steinacker, P., Hendrich, C., Sperfeld, A. D., Jesse, S., Lehnert, S., Pabst, A., et al. (2009). Concentrations of beta-amyloid precursor protein processing products in cerebrospinal fluid of patients with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Journal of Neural Transmission,116(9), 1169–1178.
Tamagno, E., Parola, M., Bardini, P., Piccini, A., Borghi, R., Guglielmotto, M., et al. (2005). Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. Journal of Neurochemistry,92(3), 628–636.
Tarkowski, E., Andreasen, N., Tarkowski, A., & Blennow, K. (2003). Intrathecal inflammation precedes development of Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry,74(9), 1200–1205.
Taverna, M., Straub, T., Hampel, H., Rujescu, D., & Lichtenthaler, S. F. (2013). A new sandwich immunoassay for detection of the α-secretase cleaved, soluble amyloid-β protein precursor in cerebrospinal fluid and serum. Journal of Alzheimer’s disease,37(4), 667–678.
Tezapsidis, N., Li, H. C., Ripellino, J. A., Efthimiopoulos, S., Vassilacopoulou, D., Sambamurti, K., et al. (1998). Release of nontransmembrane full-length Alzheimer’s amyloid precursor protein from the lumenar surface of chromaffin granule membranes. Biochemistry,37(5), 1274–1282.
Tsolakidou, A., Alexopoulos, P., Guo, L. H., Grimmer, T., Westerteicher, C., Kratzer, M., et al. (2013). β-Site amyloid precursor protein-cleaving enzyme 1 activity is related to cerebrospinal fluid concentrations of sortilin-related receptor with A-type repeats, soluble amyloid precursor protein, and tau. Alzheimer’s and Dementia,9(4), 386–391.
van Waalwijk van Doorn, L. J., Koel-Simmelink, M. J., Haußmann, U., Klafki, H., Struyfs, H., Linning, P., et al. (2016). Validation of soluble amyloid-β precursor protein assays as diagnostic CSF biomarkers for neurodegenerative diseases. Journal of Neurochemistry,137(1), 112–121.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. (2011) Tugwell The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [online]. The Ottawa Hospital Rresearch Institute. Available at: http://www.ohri.ca/programs/clinical epidemiology/oxford.asp. Accessed May 2, 2019.
Zetterberg, H., Andreasson, U., Hansson, O., Wu, G., Sankaranarayanan, S., Andersson, M. E., et al. (2008). Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Archives of Neurology,65(8), 1102–1107.
Acknowledgments
This work was funded in part by the Anhui Provincial Natural Science Foundation, Key Research and Development Plan “A” (Grant Numbers 1804h08020236); the Key Project of University Natural Science Foundation of Anhui Province (Grant Numbers KJ2018A0206).
Funding
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
The manuscript is a retrospective report that does not require ethics committee approval at our institution.
Informed Consent
Written informed consent was obtained from all participants involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, W., Wang, Y., Cheng, J. et al. CSF sAPPα and sAPPβ levels in Alzheimer’s Disease and Multiple Other Neurodegenerative Diseases: A Network Meta-Analysis. Neuromol Med 22, 45–55 (2020). https://doi.org/10.1007/s12017-019-08561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-019-08561-7