Abstract
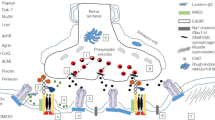
Congenital myasthenic syndromes (CMS) are heterogeneous genetic diseases in which neuromuscular transmission is compromised. CMS resembling the Lambert–Eaton myasthenic syndrome (CMS–LEMS) are emerging as a rare group of distinct presynaptic CMS that share the same electrophysiological features. They have low compound muscular action potential amplitude that increment after brief exercise (facilitation) or high-frequency repetitive nerve stimulation. Although clinical signs similar to LEMS can be present, the main hallmark is the electrophysiological findings, which are identical to autoimmune LEMS. CMS–LEMS occurs due to deficits in acetylcholine vesicle release caused by dysfunction of different components in its pathway. To date, the genes that have been associated with CMS–LEMS are AGRN, SYT2, MUNC13-1, VAMP1, and LAMA5. Clinicians should keep in mind these newest subtypes of CMS–LEMS to achieve the correct diagnosis and therapy. We believe that CMS–LEMS must be included as an important diagnostic clue to genetic investigation in the diagnostic algorithms to CMS. We briefly review the main features of CMS–LEMS.


Similar content being viewed by others
References
Aran, A., Segel, R., Kaneshige, K., et al. (2017). Vesicular acetylcholine transporter defect underlies devastating congenital myasthenia syndrome. Neurology, 88, 1021–1028.
Bady, D., Chauplannaz, G., & Carrier, H. (1987). Congenital Lambert-Eaton myasthenic syndrome. Journal of Neurology, Neurosurgery, and Psychiatry, 50, 476–478.
Beeson, D., Hantaï, D., Lochmüller, H., & Engel, A. G. (2005). 126th International Workshop: congenital myasthenic syndromes, 24–26 September 2004, Naarden, The Netherlands. Neuromuscular Disorders, 15, 498–512.
Campagna, J. A., Ruegg, M. A., & Bixby, J. L. (1997). Evidence that agrin directly influences presynaptic differentiation at neuromuscular junctions in vitro. European Journal of Neuroscience, 9, 2269–2283.
Engel, A. G., Ohno, K., & Sine, S. M. (2003). Congenital myasthenic syndromes: Progress over the past decade. Muscle and Nerve, 27, 4–25.
Engel, A. G., Selcen, D., Shen, X. M., Milone, M., & Harper, C. M. (2016). Loss of MUNC13-1 function causes microcephaly, cortical hyperexcitability, and fatal myasthenia. Neurology Genetics, 2, e105.
Engel, A. G., Shen, X. M., & Selcen, D. (2018). The unfolding landscape of the congenital myasthenic syndromes. Annals of the New York Academy of Sciences, 1413, 25–34.
Han, J., Pluhackova, K., & Böckmann, R. A. (2017). The multifaceted role of SNARE proteins in membrane fusion. Frontiers in Physiology, 8, 5.
Herrmann, D. N., Horvath, R., Sowden, J. E., et al. (2014). Synaptotagmin 2 mutations cause an autosomal-dominant form of Lambert-Eaton myasthenic syndrome and nonprogressive motor neuropathy. American Journal of Human Genetics, 95, 332–339.
Hülsbrink, R., & Hashemolhosseini, S. (2014). Lambert-Eaton myasthenic syndrome: diagnosis, pathogenesis and therapy. Clinical Neurophysiology, 125, 2328–2336.
Jahn, R., & Fasshauer, D. (2012). Molecular machines governing exocytosis of synaptic vesicles. Nature, 490, 201–207.
Lorenzoni, P. J., Scola, R. H., Kay, C. S., Parolin, S. F., & Werneck, L. C. (2010). Non-paraneoplastic Lambert-Eaton myasthenic syndrome: A brief review of 10 cases. Arquivos de Neuro-Psiquiatria, 68, 849–854.
Lorenzoni, P. J., Scola, R. H., Kay, C. S. K., & Werneck, L. C. (2012). Congenital myasthenic syndrome: A brief review. Pediatric Neurology, 46, 141–148.
Ma, C., Su, L., Seven, A. B., Yu, Y., & Rizo, J. (2013). Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science, 339, 421–425.
Malsam, J., Kreye, S., & Sollner, T. H. (2008). Membrane fusion: SNAREs and regulation. Cellular and Molecular Life Sciences, 65, 2814–2832.
Maselli, R. A., Arredondo, J., Ferns, M. J., & Wollmann, R. L. (2012). Synaptic basal lamina-associated congenital myasthenic syndromes. Annals of the New York Academy of Sciences, 1275, 36–48.
Maselli, R. A., Arredondo, J., Vazquez, J., et al. (2017). Presynaptic congenital myasthenic syndrome with a homozygous sequence variant in LAMA5 combines myopia, facial tics, and failure of neuromuscular transmission. American Journal of Medical Genetics, 173, 2240–2245.
Maselli, R. A., Fernandez, J. M., Arredondo, J., et al. (2012). LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Human Genetics, 131, 1123–1135.
McMacken, G., Abicht, A., Evangelista, T., Spendiff, S., & Lochmüller, H. (2017). The increasing genetic and phenotypical diversity of congenital myasthenic syndromes. Neuropediatrics, 48, 294–308.
Miner, J. H., Cunningham, J., & Sanes, J. R. (1998). Roles for laminin in embryogenesis: Exencephaly, syndactyly, and placentopathy in mice lacking the laminin-5 chain. Journal of Cell Biology, 143, 1713–1723.
Nicole, S., Azuma, Y., Bauché, S., Eymard, B., Lochmüller, H., & Slater, C. (2017). Congenital myasthenic syndromes or inherited disorders of neuromuscular transmission: Recent discoveries and open questions. Journal of Neuromuscular Diseases, 4, 269–284.
Nicole, S., Chaouch, A., Torbergsen, T., et al. (2014). Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain, 137, 2429–2443.
Nishimune, H., Sanes, J. R., & Carlson, S. S. (2004). A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature, 432, 580–587.
Rizo, J., & Xu, J. (2015). The synaptic vesicle release machinery. Annual Review of Biophysics, 44, 339–367.
Salpietro, V., Lin, W., Vedove, A. D., et al. (2017). Homozygous mutations in VAMP1 cause a presynaptic congenital myasthenic syndrome. Annals of Neurology, 81, 597–603.
Schoser, B., Eymard, B., Datt, J., & Mantegazza, R. (2017). Lambert-Eaton myasthenic syndrome (LEMS): A rare autoimmune presynaptic disorder often associated with cancer. Journal of Neurology, 264, 1854–1863.
Shen, X. M., Scola, R. H., Lorenzoni, P. J., et al. (2017). Novel synaptobrevin-1 mutation causes fatal congenital myasthenic syndrome. Annals of Clinical and Translational Neurology, 4, 130–138.
Shen, X. M., Selcen, D., Brengman, J., & Engel, A. G. (2014). Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology, 83, 2247–2255.
Son, Y. J., Scranton, T. W., Sunderland, W. J., et al. (2000). The synaptic vesicle protein SV2 is complexed with an alpha5-containing laminin on the nerve terminal surface. Journal of Biological Chemistry, 275, 451–460.
Souza, P. V., Batistella, G. N., Lino, V. C., Pinto, W. B., Annes, M., & Oliveira, A. S. (2016). Clinical and genetic basis of congenital myasthenic syndromes. Arquivos de Neuro-Psiquiatria, 4, 750–760.
Sudhof, T. C., & Rothman, J. E. (2009). Membrane fusion: grappling with SNARE and SM proteins. Science, 323, 474–477.
Titulaer, M. J., Lang, B., & Verschuuren, J. (2011). Lambert–Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurology, 10, 1098–1107.
Whittaker, R. G., Herrmann, D. N., Bansagi, B., et al. (2015). Electrophysiologic features of SYT2 mutations causing a treatable neuromuscular syndrome. Neurology, 85, 1964–1971.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Lorenzoni, P.J., Scola, R.H., Kay, C.S.K. et al. How to Spot Congenital Myasthenic Syndromes Resembling the Lambert–Eaton Myasthenic Syndrome? A Brief Review of Clinical, Electrophysiological, and Genetics Features. Neuromol Med 20, 205–214 (2018). https://doi.org/10.1007/s12017-018-8490-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-018-8490-1