Abstract
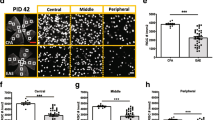
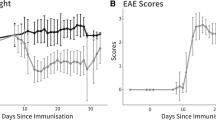
Optic neuritis associated with multiple sclerosis and its animal model, experimental autoimmune optic neuritis (EAON), is characterized by inflammation, T cell activation, demyelination, and neuronal damage, which might induce permanent vision loss. Elucidating the chronological relationship among the features is critical for treatment of demyelinating optic neuritis. EAON was induced in C57BL/6 mice immunized with myelin oligodendrocyte glycoprotein subcutaneously, and visual function was assessed by flash-visual evoked potential (F-VEP) at days 7, 11, 14, 19, 23, 28 post-immunization. Retinal ganglion cell (RGC) apoptosis was measured by terminal-deoxynucleotidyl transferase-mediated nick-end labeling. Demyelination and axonal damage were verified with myelin basic protein (MBP) and β-amyloid precursor protein staining, respectively. Real-time polymerase chain reaction quantified IL-17, IL-1β, TGF-β, FoxP3, IL-6, and IL-10 mRNA expression in the optic nerve, as well as FoxP3 and IL-17 staining. Systemic changes of Th17 and Treg cells were tested by flow cytometry in spleen. F-VEP latency was prolonged at 11 days and peaked at 23 days commensurate with demyelination. However, F-VEP amplitude was reduced at 11 days, preceding axon damage, and was exacerbated at 23 days when a peak in RGC apoptosis was detected. Th17 cells up-regulated as early as 7 days and peaked at 11 days, while Treg cells down-regulated inversely compared to Th17 cells change as verified by IL-17 and FoxP3 expression; spleen cell samples were slightly different, demonstrating marked changed at 14 days. Treg/Th17 cell imbalance in the optic nerve precedes and may initiate neuronal damage of axons and RGCs. These changes are commensurate with the appearances of visual dysfunction reflected in F-VEP and hence may offer a novel therapeutic avenue for vision preservation.










Similar content being viewed by others
References
Aranami, T., & Yamamura, T. (2008). Th17 cells and autoimmune encephalomyelitis (EAE/MS). Allergology International, 57(2), 115–120.
Bettelli, E., Carrier, Y., Gao, W., et al. (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature, 441, 235–238.
Bilbool, N., Kaitz, M., Feinsod, M., et al. (1983). Visual evoked potentials in experimental allergic encephalomyelitis. Journal of the Neurological Sciences, 60(1), 105–115.
Diem, R., Tschirne, A., & Bähr, M. (2003). Decreased amplitudes in multiple sclerosis patients with normal visual acuity: A VEP study. Journal of Clinical Neuroscience, 10, 67–71.
Dutt, M., Rostami, A., Shindler, K. S., et al. (2010). Timing of corticosteroid therapy is critical to prevent retinal ganglion cell loss in experimental optic neuritis. Investigative Ophthalmology & Visual Science, 51(3), 1439–1445.
Flugel, A., Berkowicz, T., Ritter, T., et al. (2001). Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity, 14, 547.
Furlan, R., Cuomo, C., & Martino, G. (2009) Animal models of multiple sclerosis. Methods in Molecular Biology, 549, 157–173.
Garcia-Martin, E., Polo, V., Larrosa, J. M., et al. (2014). Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology, 121(2), 573–579.
Gold, R., Linington, C., & Lassmann, H. (2006). Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis re-search. Brain, 129, 1953–1971.
Guan, Y., Shindler, K. S., Tabuena, P., et al. (2006). Retinal ganglion cell damage induced by spontaneous autoimmune optic neuritis in MOG-specific TCR transgenic mice. Journal of Neuroimmunology, 178(1–2), 40–48.
Halliday, A., McDonald, W., & Mushin, J. (1972). Delayed visual evoked response in optic neuritis. Lancet, 1, 982–985.
Hobom, M., Storch, M. K., Weissert, R., et al. (2004). Mechanisms and time course of neuronal degeneration in experimental autoimmune encephalomyelitis. Brain Pathology, 14(2), 148–157.
Horstmann, L., Schmid, H., Heinen, A. P., et al. (2013). Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. Journal of Neuroinflammation, 4(10), 120.
Kebir, H., Kreymborg, K., Ifergan, I., et al. (2007). Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nature Medicine, 13(10), 1173–1175.
Klistorner, A., Graham, S., Fraser, C., et al. (2007). Electrophysiological evidence for heterogeneity of lesions in optic neuritis. Investigative Ophthalmology & Visual Science, 48, 4549–4556.
Kuhlmann, T., Lingfeld, G., Bitsch, A., et al. (2002). Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain, 125(Pt 10), 2202–2212.
Kuroiwa, Y., & Shibasaki, H. (1973) Clinical studies of multiple sclerosis in Japan. I. A current appraisal of 83 cases. Neurology, 23(6), 609–617.
Leibowitz, U., & Alter, M. (1968). Optic nerve involvement and diplopia as initial manifestations of multiple sclerosis. Acta Neurologica Scandinavica, 44, 70–80.
Lock, C., Hermans, G., Pedotti, R., et al. (2002). Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature Medicine, 8(5), 500–508.
Matsuda, R., Kezuka, T., Nishiyama, C., et al. (2012). Interleukin-10 gene-transfected mature dendritic cells suppress murine experimental autoimmune optic neuritis. Investigative Ophthalmology & Visual Science, 53, 7235–7245.
Matsunaga, Y., Kezuka, T., An, X., et al. (2012). Visual functional and histopathological correlation in experimental autoimmune optic neuritis. Investigative Ophthalmology & Visual Science, 53(11), 6964–6971.
McAllister, F., Henry, A., Kreindler, J. L., et al. (2005). Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: Implications or airway inflammation in cystic fibrosis. The Journal of Immunology, 175, 404–412.
Meyer-Franke, A., Wilkinson, G. A., Kruttgen, A., et al. (1998). Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron, 21, 681–693.
Narayanan, D., Cheng, H., Bonem, K. N. et al. (2014) Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler. [Epub ahead of print].
Oukka, M. (2007). Interplay between pathogenic Th17 and regulatory T cells. Annals of the Rheumatic Diseases, 66(Suppl 3), iii87–iii90.
Prilloff, S., Henrich-Noack, P., & Sabel, B. A. (2012). Recovery of axonal transport after partial optic nerve damage is associated with secondary retinal ganglion cell death in vivo. Investigative Ophthalmology & Visual Science, 53(3), 1460–1466.
Quinn, T. A., Dutt, M., & Shindler, K. S. (2011). Optic neuritis and retinal ganglion cell loss in a chronic murine model of multiple sclerosis. Frontiers in neurology, 2, 50.
Sabel, B. A. (1999). Restoration of vision I: Neurobiological mechanisms of restoration and plasticity after brain damage—A review. Restorative Neurology and Neuroscience, 15(2–3), 177–200.
Sabel, B. A. (2008). Plasticity and restoration of vision after visual system damage: An update. Restorative Neurology and Neuroscience, 26(4–5), 243–247.
Sättler, M. B., Williams, S. K., Diem, R., et al. (2008). Flupirtine as neuroprotective add-on therapy in autoimmune optic neuritis. American Journal of Pathology, 173(5), 1496–1507.
Shao, H., Huang, Z., Sun, S. L., et al. (2004). Myelin/oligodendrocyte glycoprotein-specific T-cells induce severe optic neuritis in the C57BL/6 mouse. Investigative Ophthalmology & Visual Science, 45, 4060–4065.
Shields, D. C., Schaecher, K. E., Saido, T. C., et al. (1999). A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proceedings of the National Academy of Sciences of the United States of America, 96, 11486–11491.
Shindler, K. S., Ventura, E., Dutt, M., et al. (2008). Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Experimental Eye Research, 87(3), 208–213.
Sorensen, T. L., Frederiksen, J. L., Bronnum-Hansen, H., et al. (1999). Optic neuritis as onset manifestation of multiple sclerosis: A nationwide, long-term survey. Neurology, 53, 473–478.
Touil, T., Ciric, B., Shindler, K. S., et al. (2008). Bowman–Birk inhibitor suppresses autoimmune inflammation and neuronal loss in a mouse model of multiple sclerosis. Journal of the Neurological Sciences, 271(1–2), 191–202.
Trapp, B. D., Ransohoff, R., & Rudick, R. (1999). Axonal pathology in multiple sclerosis: Relationship to neurologic disability. Current Opinion in Neurology, 12, 295–302.
Verhagen, J., Burton, B. R., Britton, G. J., et al. (2013). Modification of the FoxP3 transcription factor principally affects inducible T regulatory cells in a model of experimental autoimmune encephalomyelitis. PLoS One, 8(4), e61334.
Walter, S. D., Ishikawa, H., Galetta, K. M., et al. (2012). Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology, 119(6), 1250–1257.
Xiao, J., Liu, C., Li, G., et al. (2013). PDCD5 negatively regulates autoimmunity by upregulating FOXP3(+) regulatory T cells and suppressing Th17 and Th1 responses. Journal of Autoimmunity, 47, 34–44.
Yan, H., Li, F., & Zhang, L. (2012). A new and reliable animal model for optic nerve injury. Current Eye Research, 37(10), 941–948.
You, Y., Klistorner, A., Thie, J., et al. (2011). Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Investigative Ophthalmology & Visual Science, 52(9), 6911–6918.
Acknowledgments
This study was supported by Natural Science Foundation of Tianjin grant (Grant Numbers 12JCYBJC33900 and 14JCYBJC28000) and National Natural Science Foundation of China (Grant Numbers 81371038 and 91442124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., You, C., Zhang, Z. et al. Roles of Treg/Th17 Cell Imbalance and Neuronal Damage in the Visual Dysfunction Observed in Experimental Autoimmune Optic Neuritis Chronologically. Neuromol Med 17, 391–403 (2015). https://doi.org/10.1007/s12017-015-8368-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-015-8368-4