Abstract
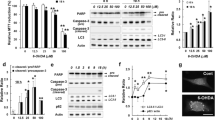
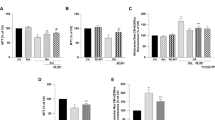
In the present study, we tried to answer the following questions: which kind of defense pathways are activated after Aβ insult? How defense systems react against noxious effects of Aβ and whether they are able to deal against apoptosis or not? So, we traced some molecular pathways including autophagy, mitophagy, and mitochondrial biogenesis before reaching to the endpoint of apoptosis. Besides, we measured the function of mitochondria after injection of Aβ (1–42) in CA1 area of hippocampus as a model of Alzheimer’s disease (AD). Based on our data, autophagy markers reached to their maximum level and returned to the control level as apoptotic markers started to increase. As a specialized form of autophagy, mitophagy markers followed the trend of autophagy markers. Whereas mitochondrial dynamic processes shifted toward fission, mitochondrial biogenesis was severely affected by Aβ and significantly decreased. Alongside suppression of mitochondrial biogenesis, activity of specific enzymes involved in antioxidant defense system, electron transport chain, and tricarboxylic acid cycle (TCA) decreased in response to the Aβ. Activity of antioxidant enzymes increased at first and then decreased significantly compared to the control. TCA enzymes aconitase and malate dehydrogenase activities reduced immediately while citrate synthase and fumarase activities did not change. Based on our finding, monitoring of the master molecules of intracellular cascades and determining their trends before the destructive function of Aβ could be the target of therapeutic issues for AD.







Similar content being viewed by others
References
Adam-Vizi, V., & Chinopoulos, C. (2006). Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends in Pharmacological Sciences, 27(12), 639–645.
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Ashrafi, G., & Schwarz, T. L. (2013). The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differentiation, 20(1), 31–42.
Birch-Machin, M. A., Shepherd, I. M., Watmough, N. J., Sherrat, H. S., Bartlett, K., Darley-Usmar, V. M., et al. (1989). Fatal lactic acidosis in infancy with a defect of complex III of the respiratory chain. Pediatric Research, 25, 553–559.
Boengler, K., Heusch, G., & Schulz, R. (2011). Nuclear-encoded mitochondrial proteins and their role in cardioprotection. Biochimica et Biophysica Acta, 1813(7), 1286–1294.
Braak, H., & Braak, E. (1991). Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathology, 1(3), 213–216.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bubber, P., Haroutunian, V., Fisch, G., Parvesh, B., & Vahram, H. (2005). Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Annals of Neurology, 57, 695–703.
Chen, H., Chan, D. C. (2009). Mitochondrial dynamics—Fusion, fission, movement, and mitophagy—In neurodegenerative diseases. Human Molecular Genetics, 18 (R2), R169–R176.
Christen, Y. (2000). Oxidative stress and Alzheimer disease. The American Journal of Clinical Nutrition, 71(2), 621S–629S.
Cipolat, S., Martins de Brito, O., Dal Zilio, B., & Scorrano, L. (2004). OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proceedings of the National Academy of Sciences, 101(45), 15927–15932.
Clarke, J. B., & Nicklas, W. J. (1970). The metabolism of rat brain mitochondria preparation and characterization. Journal of Biological Chemistry, 245(18), 4724–4731.
Cottingham, M. G., Hollinshead, M. S., & Vaux, D. J. (2002). Amyloid fibril formation by a synthetic peptide from a region of human acetylcholinesterase that is homologous to the Alzheimer’s amyloid-beta peptide. Biochemistry, 41(46), 13539–13547.
Cunningham, J. T., Rodgers, J. T., Arlow, D. H., Vazquez, F., Mootha, V. K., & Puigserver, P. (2007). mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature, 450(7170), 736–740.
Darios, F., Corti, O., Lucking, C. B., Hampe, C., Muriel, M. P., Abbas, N., et al. (2003). Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Human Molecular Genetics, 12, 517–526.
De Duve, C., & Wattiaux, R. (1966). Functions of lysosomes. Annual Review of Physiology, 28, 435–492.
Eisenberg-Lerner, A., Bialik, S., Simon, H.-U., & Kimchi, A. (2009). Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death and Differentiation, 16, 966–975.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70–77.
Flint, D. H., Tuminello, J. F., & Emptage, M. H. (1993). The inactivation of Fe–S cluster containing hydro-lyases by superoxide. Journal of Biological Chemistry, 268, 22369–22376.
Fujita, N., Noda, T., & Yoshimori, T. (2009). Atg4BC74A hampers autophagosome closure: A useful protein for inhibiting autophagy. Autophagy, 5(1), 88–89.
Gardner, P. R., Raineri, I., Epstein, L. B., & White, C. W. (1995). Superoxide radical and iron modulate aconitase activity in mammalian cells. Journal of Biological Chemistry, 270, 13399–13405.
Glenner, G. G., & Wong, C. W. (1984). Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and Biophysical Research Communications, 120, 885–890.
Gleyzer, N., Vercauteren, K., & Scarpulla, R. C. (2005). Control of mitochondrial transcription specificity factors (TFB1 M and TFB2 M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Molecular Cell Biology, 25(4), 1354–1366.
Goldberg, A. L. (2003). Protein degradation and protection against misfolded or damaged proteins. Nature, 426, 895–899.
Haass, C., & Selkoe, D. J. (2007). Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid b-peptide. Nature Reviews Molecular Cell Biology, 8, 101–112.
Hara, T., Nakamura, K., Matsui, M., Yamamoto, A., Nakahara, Y., Suzuki-Migishima, R., et al. (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature, 441, 885–889.
Heinonen, H., Nieminen, A., Saarela, M., Kallioniemi, A., Klefström, J., Hautaniemi, S., et al. (2008). Deciphering downstream gene targets of PI3 K/mTOR/p70S6 K pathway in breast cancer. BMC Genomics, 2008(9), 348–360.
Hui Bon Hoa, G., McLean, M. A., & Sligar, S. G. (2002). High pressure, a tool for exploring heme protein active sites. Biochimica et Biophysica Acta, 1595(1–2), 297–308.
Jones, D. P. (2002). Redox potential of GSH/GSSG couple: Assay and biological significance. Methods in Enzymology, 348, 93–112.
Jones, C. W., & Poole, R. K. (1985). The analysis of cytochromes. Methods of microbiology, 18, 285–328.
Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry & Biophysics, 21, 130–132.
Kim, H. C., Yamada, K., Nitta, A., Olariu, A., Tran, M. H., Mizuno, M., et al. (2003). Immunocytochemical evidence that amyloid beta (1–42) impairs endogenous antioxidant systems in vivo. Neuroscience, 119, 399–419.
Klionsky, D. J., Cregg, J. M., Dunn, W. A., Jr, Emr, S. D., Sakai, Y., Sandoval, I. V., et al. (2003). A unified nomenclature for yeast autophagy-related genes. Developmental Cell, 5, 539–545.
Kohler, J. J., Cucoranu, I., Fields, E., Green, E., He, S., Hoying, A., et al. (2009). Transgenic mitochondrial superoxide dismutase and mitochondrially targeted catalase prevent antiretroviral-induced oxidative stress and cardiomyopathy. Laboratory Investigation, 89(7), 782–790.
Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J., Tanida, I., et al. (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature, 441, 880–884.
Lamark, T., Kirkin, V., Dikic, I., & Johansen, T. (2009). NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle, 8(13), 1986–1990.
Levine, B., & Kroemer, G. (2008). Autophagy in the pathogenesis of disease. Cell, 132, 27–42.
Li, W., Yang, Q., & Mao, Z. (2011). Chaperone-mediated autophagy: machinery, regulation and biological consequences. Cellular and Molecular Life Sciences, 68, 749–763.
Manczak, M., Park, B. S., Jung, Y., & Reddy, P. H. (2004). Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: Implications for early mitochondrial dysfunction and oxidative damage. NeuroMolecular Medicine, 5, 147–162.
Minoshima, S., Giordani, B., Berent, S., Frey, K. A., Foster, N. L., & Kuhl, D. E. (1997). Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Annals of Neurology, 42, 85–94.
Morán, M., Moreno-Lastres, D., Marín-Buera, L., Arenas, J., Martín, M. A., & Ugalde, C. (2012). Mitochondrial respiratory chain dysfunction: Implications in neurodegeneration. Free Radical Biology & Medicine, 53, 595–609.
Morishima, Y., Gotoh, Y., Zieg, J., Barrett, T., Takano, H., Flavell, R., et al. (2001). β-Amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-Terminal kinase pathway and the induction of Fas ligand. Journal of Neuroscience, 21(19), 7551–7560.
Morishima-Kawashima, M., & Ihara, Y. (2002). Alzheimer’s disease: Beta-Amyloid protein and tau. Journal of Neuroscience Research, 70(3), 392–401.
Muller, F. L., Liu, Y., & Van Remmen, H. (2004). Complex III releases superoxide to both sides of the inner mitochondrial membrane. Journal of Biological Chemistry, 279, 49064–49073.
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochemical Journal, 417(Pt 1), 1–13.
Narendra, D. P., & Youle, R. J. (2011). Targeting mitochondrial dysfunction: Role for PINK1 and Parkin in mitochondrial quality control. Antioxidants & Redox Signaling, 14(10), 1929–1938.
Patel, M., Day, B. J., Crapo, J. D., Fridovich, I., & McNamara, J. O. (1996). Requirement for superoxide in excitotoxic cell death. Neuron, 16, 345–355.
Paxinos, G., & Watson, C. (2007). The rat brain in stereotaxic coordinates. Amsterdam: Elsevier Academic press.
Powell, C. S., & Jackson, R. M. (2003). Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: Modulation of enzyme activities by MnSOD. American Journal of Physiology, 285(1), L189–L198.
Qi, Z., & Ding, S. (2012). Transcriptional regulation by nuclear corepressors and PGC-1α: Implications for mitochondrial quality control and insulin sensitivity. PPAR Research, 2012, 1–12.
Racker, E. (1950). Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochimica et Biophysica Acta, 4(1–3), 211–214.
Ravikumar, B., Futter, M., Jahreiss, L., Korolchuk, V. I., Lichtenberg, M., Luo, S., et al. (2009). Mammalian macroautophagy at a glance. Journal of Cell Science, 122, 1707–1711.
Rhein, V., Baysang, G., Rao, S., Meier, F., Bonert, A., Muller-Spahn, F., et al. (2009). Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma Cells. Cellular and Molecular Neurobiology, 29, 1063–1071.
Rubinsztein, D. C. (2006). The roles of intracellular protein-degradation pathways in neurodegeneration. Nature, 443, 780–786.
Rustin, P., Chretien, D., Bourgeron, T., Wucher, A., Saudubray, J. M., Rotig, A., et al. (1991). Assessment of the mitochondrial respiratory chain. Lancet, 338, 60.
Sahu, R., Kaushik, S., Clement, C. C., Cannizzo, E. S., Scharf, B., Follenzi, A., et al. (2011). Microautophagy of cytosolic proteins by late endosomes. Developmental Cell, 20(3), 405–406.
Saikumar, P., Dong, Z., Mikhailov, V., Denton, M., Weimberg, J. M., & Venkatachalam, M. A. (1999). Apoptosis: Definition, mechanisms, and relevance to disease. American Journal of Medicine, 107(5), 489–506.
Santos, R. X., Correia, S. C., Wang, X., Perry, G., Smith, M. A., Moreira, P. I., et al. (2010). A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer’s disease. Journal of Alzheimers Disease, 2, S401–S412.
Sazanov, L. A. (2007). Respiratory complex I: Mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry, 46, 2275–2288.
Scarpulla, R. C. (2002). Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochimica et Biophysica Acta, 1576(1–2), 1–14.
Scarpulla, R. C. (2011). Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochimica et Biophysica Acta, 1813(7), 1269–1278.
Schweichel, J. U., & Merker, H. J. (1973). The morphology of various types of cell death in prenatal tissues. Teratology, 7, 253–266.
Small, D. H., Gasperini, R., Vincent, A. J., Hung, A. C., & Foa, L. (2009). The role of Abeta-induced calcium dysregulation in the pathogenesis of Alzheimer’s disease. Journal of Alzheimers Disease, 16, 225–233.
Srere, P. A. (1974). Controls of citrate synthase. Life. Science, 15, 1695–1710.
Stadelmann, C., Deckwerth, T. L., Srinivasan, A., Bancher, C., Brück, W., Jellinger, K., et al. (1999). Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. American Journal of Pathology, 155(5), 1459–1466.
Tanida, I. (2011). Autophagy basics. Microbiology and Immunology, 55, 1–11.
Troy, C. M., Rabacchi, S. A., Friedman, W. J., Frappier, T. F., Brown, K., & Shelanski, M. L. (2000). Caspase-2 mediates neuronal cell death induced by β-amyloid. Journal of Neuroscience, 20(4), 1386–1392.
Tuppoa, E. E., & Arias, H. R. (2009). The role of inflammation in Alzheimer’s disease. International Journal of Biochemistry & Cell Biology, 37, 289–305.
Veereshwarayya, V., Kumar, P., Rosen, K. M., Mestril, R., & Querfurth, H. W. (2006). Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular β-amyloid-induced inhibition of complex IV and limit apoptosis. Journal of Biological Chemistry, 281, 29468–29478.
Vitolo, O. V., Sant’Angelo, A., Costanzo, V., Battaglia, F., Arancio, O., & Shelanski, M. (2002). Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proceedings of the National Academy of Sciences, 99(20), 13217–13221.
Wang, L., Mascher, H., Psilander, N., Blomstrand, E., & Sahlin, K. (2011). Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. Journal of Applied Physiology, 111, 1335–1344.
Wang, Q., Yu, L., & Yu, C. A. (2010). Cross-talk between mitochondrial malate dehydrogenase and the cytochrome bc1 complex. Journal of Biological Chemistry, 285(14), 10408–10414.
Watson, D., Castano, E., Kokjohn, T. A., Kuo, Y. M., Lyubchenko, Y., et al. (2005). Physicochemical characteristics of soluble oligomeric Aβ and their pathologic role in Alzheimer’s disease. Journal of Neurology Research, 27, 869–881.
Wenz, T. (2013). Regulation of mitochondrial biogenesis and PGC-1α under cellular stress. Mitochondrion, 13(2), 134–142.
Yan, S. D., Chen, X., Fu, J., Chen, M., & Zhu, H. (1996). RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature, 382, 685–691.
Yu, W. H., Cuervo, A. M., Kumar, A., Peterhoff, C. M., Schmidt, S. D., Lee, J. H., et al. (2005). Macroautophagy—A novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. Journal of Cell Biology, 171(1), 87–98.
Yu, L., & Yang, S. J. (2010). AMP-activated protein kinase mediates activity-dependent regulation of peroxisome proliferator-activated receptor gamma coactivator-1alpha and nuclear respiratory factor 1 expression in rat visual cortical neurons. Neuroscience, 169(1), 23–38.
Zheng, W., Ren, S., & Graziano, J. H. (1998). Manganese inhibits mitochondrial aconitase: A mechanism of manganese neurotoxicity. Brain Reseach, 799(2), 334–342.
Zhu, J., Wang, K. Z. Q., & Chu, C. T. (2013). After the banquet: Mitochondrial biogenesis, mitophagy and cell survival. Autophagy, 9(9), 1–14.
Acknowledgments
This work is part of PhD student thesis of F. Shaerzadeh at the Shahid Beheshti University of Medical Sciences.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaerzadeh, F., Motamedi, F., Minai-Tehrani, D. et al. Monitoring of Neuronal Loss in the Hippocampus of Aβ-Injected Rat: Autophagy, Mitophagy, and Mitochondrial Biogenesis Stand Against Apoptosis. Neuromol Med 16, 175–190 (2014). https://doi.org/10.1007/s12017-013-8272-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8272-8