Abstract
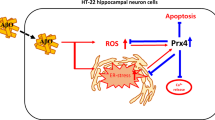
We have previously shown the involvement of p66shc in mediating apoptosis. Here, we demonstrate the novel mechanism of β-Amyloid-induced toxicity in the mammalian cells. β-Amyloid leads to the phosphorylation of p66shc at the serine 36 residue and activates MKK6, by mediating the phosphorylation at serine 207 residue. Treatment of cells with antioxidants blocks β-Amyloid-induced serine phosphorylation of MKK6, reactive oxygen species (ROS) generation, and hence protected cells against β-Amyloid-induced cell death. Our results indicate that serine phosphorylation of p66shc is carried out by active MKK6. MKK6 knock-down resulted in decreased serine 36 phosphorylation of p66shc. Co-immunoprecipitation results demonstrate a direct physical association between p66shc and WT MKK6, but not with its mutants. Increase in β-Amyloid-induced ROS production was observed in the presence of MKK6 and p66shc, when compared to triple mutant of MKK6 (inactive) and S36 mutant of p66shc. ROS scavengers and knock-down against p66shc, and MKK6 significantly decreased the endogenous level of active p66shc, ROS production, and cell death. Finally, we show that the MKK6–p66shc complex mediates β-Amyloid-evoked apoptotic cell death.








Similar content being viewed by others
References
Bashir, M., Kirmani, D., Bhat, H. F., Baba, R. A., Hamza, R., Naqash, S., et al. (2010). P66shc and its associate targets are upregulated in esophageal cancers. Cell Communication and Signaling, 8, 13.
Brera, B., Serrano, A., & De Ceballos, M. L. (2000). β-amyloid peptides are cytotoxic to astrocytes in culture: A role for oxidative stress. Neurobiology of Disease, 7, 395–405.
Canevari, L., Abramov, A. Y., & Duchen, M. R. (2004). Toxicity of amyloid peptide: Tales of calcium, mitochondria, and oxidative stress. Neurochemistry and Research, 29, 637–650.
Corrêa, S. A. L., & Eales, K. L. (2012). The role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative diseases. Journal of Signal transduction, 2012, 1–12.
Cotman, C. W., Whittemore, E. R., Watt, J. A., Anderson, A. J., & Loo, D. T. (1994). Possible role of apoptosis in Alzheimer’s disease. Annuals of New York Acadamy Sciences, 747, 36–49.
Das, D. K., Maulik, N., & Engelman, R. M. (2004). Redox regulation of angiotensin II signaling in the heart. Journal of Cellular and Molecular Medicine, 8, 144–152.
Derijard, B., Raingeaud, J., Barrett, T., Wu, I. H., Han, J., Ulevitch, R. J., et al. (1995). Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science, 267, 682–685.
Duyckaerts, C., Potier, M. C., & Delatour, B. (2008). Alzheimer disease models and human neuropathology: Similarities and differences. Acta Neuropathologica, 115, 5–38.
Ferrer, I., Gomez-Isla, T., Puig, B., Freixes, M., Ribe, E., Dalfo, E., et al. (2005). Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Current Alzheimer Research, 2, 3–18.
Glenner, G. G., Wong, C. W., Quaranta, V., & Eanes, E. D. (1984). The amyloid deposits in Alzheimer’s disease: Their nature and pathogenesis. Applied Pathology, 2, 357–369.
Hashimoto, Y., Tsuji, O., Niikura, T., Yamagishi, Y., Ishizaka, M., Kawasumi, M., et al. (2003). Involvement of c-Jun N-terminal kinase in amyloid precursor protein-mediated neuronal cell death. Journal of Neurochemistry, 84, 864–877.
Harraz, M. M., Park, A., Abbott, D., Zhou, W., Zhang, Y., & Engelhardt, J. F. (2007). MKK6 phosphorylation regulates production of superoxide by enhancing Rac GTPase activity. Antioxidants & Redox Signaling, 9, 1803–1813.
Hartmann, T., Bieger, S. C., Brühl, B., Tienari, P. J., Ida, N., Allsop, D., et al. (1997). Distinct sites of intracellular production for Alzheimer’s disease Aβ40/42 amyloid peptides. Nature Medicine, 3, 1016–1020.
Hensley, K., Floyd, R. A., Zheng, N. Y., Nael, R., Robinson, K. A., Nguyen, X., et al. (1999). p38 kinase is activated in the Alzheimer’s disease brain. Journal of Neurochemistry, 72, 2053–2058.
Holscher, C. (1998). Possible causes of Alzheimer’s disease: Amyloid fragments, free radicals, and calcium homeostasis. Neurobiology Disease, 5, 129–141.
Hooper, C., Killick, R., & Lovestone, S. (2008). The GSK3 hypothesis of Alzheimer’s disease. Journal of Neurochemistry, 104, 1433–1439.
Hsu, M. J., Hsu, C. Y., Chen, B. C., Chen, M. C., Ou, G., & Lin, C. H. (2007). Apoptosis signal-regulating kinase 1 in amyloid β peptide-induced cerebral endothelial cell apoptosis. Journal of Neuroscience, 27(21), 5719–5729.
Iversen, L. L., Mortishire-Smith, R. J., Pollack, S., & Shearman, M. S. (1995). The toxicity in vitro of β-amyloid protein. Biochemical Journal, 311, 1–16.
Jin, Y., Fan, Y., Yan, E. Z., Liu, Z., Zong, Z. H., & Qi, Z. M. (2006). Effects of sodium ferulate on amyloid-beta-induced MKK3/MKK6-p38 MAPK-Hsp27 signal pathway and apoptosis in rat hippocampus. Acta Pharmacologica Sinica, 27, 1309–1316.
Kalaria, R. N. (1999). Microglia and Alzheimer’s disease. Current Opinion in Hematology, 6, 15–24.
Kadowaki, H., Nishitoh, H., Urano, F., Sadamitsu, C., Matsuzawa, A., Takeda, K., et al. (2005). Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death and Differentaition, 12, 19–24.
Kawahara, M., & Kuroda, Y. (2000). Molecular mechanism of neurodegeneration induced by Alzheimer’s β-Amyloid protein: Channel formation and disruption of calcium homeostasis. Brain Research Bulletin, 53, 389–397.
Khanday, F. A., Santhanam, L., Kasuno, K., Yamamori, T., Naqvi, A., Dericio, J., et al. (2006a). SOS-mediated activation of Rac1 by p66shc. Journal of Cell Biology, 172, 817–822.
Khanday, F. A., Yamamori, T., Singh, I. M., Zhang, Z., Bugayenko, A., Naqvi, A., et al. (2006b). Rac1 Leads to Phosphorylation-dependent increase in stability of the p66shc adaptor protein: Role in rac1-induced oxidative stress. Molecular Biolpgy of Cell, 17, 122–129.
Le, S., Connors, T. J., & Maroney, A. C. (2001). c-Jun N-terminal kinase specifically phosphorylates p66ShcA at serine 36 in response to ultraviolet irradiation. Journal of Biological Chemistry, 276, 48332–48336.
Lee, D. H., & Wang, H. Y. (2003). Differential physiologic responses of alpha7 nicotinic acetylcholine receptors to β-amyloid1–40 and β-amyloid1–42. Journal of Neurobiology, 55, 25–30.
Lee, M., You, H. J., Cho, S. H., Woo, C. H., Yoo, M. H., & Joe, E. H. (2000). Implication of the small GTPase Rac1 in the generation of reactive oxygen species in response to β-amyloid in C6 astroglioma cells. Biochemistry Journal, 366, 937–943.
Li, X. D., & Buccafusco, J. J. (2003). Effect of β-Amyloid peptide 1-42 on the cytoprotective action mediated by alpha7 nicotinic acetylcholine receptors in growth factor-deprived differentiated PC-12 cells. Journal of Pharmacological Experimental Therapy, 307, 670–675.
Luzi, L., Confalonieri, S., Di Fiore, P. P., & Pelicci, P. G. (2000). Evolution of Shc functions from nematode to human. Current Opinion in Genetics & Development, 10, 668–674.
Mattson, M. P. (2002). Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer’s disease. Journal of Neurovirology, 8, 539–550.
Mattson, M. P., Barger, S. W., Cheng, B., Lieberburg, I., Smith-Swintosky, V. L., & Rydel, R. E. (1993). Β-Amyloid precursor protein metabolites and loss of Ca homeostasis in Alzheimer’s disease. Trends in Neuroscience, 16, 409–414.
Mattson, M. P., & Chan, S. L. (2003). Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium, 34, 385–397.
Migliaccio, E., Giorgio, M., Mele, S., Pelicci, G., Reboldi, P., Pandolfi, P. P., et al. (1999). The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature, 402, 309–313.
Nagele, R. G., D’Andrea, M. R., Lee, H., Venkataraman, V., & Wang, H. Y. (2003). Astrocytes accumulate Aβ42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Reearch, 971, 197–209.
Nebreda, A. R., & Porras, A. (2000). p38 MAP kinases: Beyond the stress response. Trends in Biochemical Sciences, 25, 257–260.
Nemoto, S., & Finkel, T. (2002). Redox regulation of forkhead proteins through a p66shc dependent signaling pathway. Science, 291, 2450–2452.
Okada, S., Kao, A. W., Ceresa, B. P., Blaikie, P., Margolis, B., & Pessin, J. E. (1997). The 66-kDa Shc isoform is a negative regulator of the epidermal growth factor-stimulated mitogen activated protein kinase pathway. Journal of Biological Chemistry, 272, 28042–28049.
Pacini, S., Pellegrini, M., Migliaccio, E., Patrussi, L., Ulivieri, C., Ventura, A., et al. (2004). SHC promotes apoptosis and antagonizes mitogenic signaling in T cells. Molecular and Cellular Biology, 24, 1747–1757.
Pagani, L., & Eckert, A. (2010). Amyloid-Beta Interaction with Mitochondria. International Journal of Alzheimer’s Disease, 2011, 1–12.
Pelicci, G., Lanfrancone, L., Grignani, F., McGlade, J., Cavallo, F., Forni, G., et al. (1992). A novel transforming protein (SHC) within SH2 domain is implicated in mitogenic signal transduction. Cell, 70, 93–104.
Pike, C. J., Overman, M. J., & Cotman, C. W. (1995). Amino-terminal deletions enhance aggregation of β-amyloid peptides in vitro. Journal of Biological Chemistry, 270, 23895–23898.
Purdom, S., & Chen, Q. M. (2003). P66(Shc): At the crossroad of oxidative stress and the genetics of aging. Trends in Molecular Medicine, 9, 206–210.
Rodriguez, J. J., Witton, J., Olabarria, M., Noristani, H. N., & Verkhratsky, A. (2010). Increase in the density of resting microglia precedes neuritic plaque formation and microglial activation in a transgenic model of Alzheimer’s disease. Cell Death and Disease, 1, 1–6.
Ryder, J., Su, Y., & Ni, B. (2004). Akt/GSK3β serine/threonine kinases: Evidence for a signalling pathway mediated by familial Alzheimer’s disease mutations. Cellular Signaling, 16, 187–200.
Selkoe, D. J. (1994). Alzheimer’s disease: A central role for amyloid. Journal of Neuropathology and Experimental Neurology, 53, 438–447.
Selkoe, D. J. (1999). Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature, 399(Supp), A23–A31.
Smith, W. W., Norton, D. D., Gorospe, M., Jiang, H., Nemoto, S., Holbrook, N. J., et al. (2005). Phosphorylation of p66Shc and forkhead proteins mediates Aβ Toxicity. Journal of Cell Biology, 169, 331–338.
Smith, W. W., Gorospe, M., & Kusiak, J. W. (2006). Signaling Mechanisms Underlying A beta Toxicity: Potential Therapeutic Targets for Alzheimer’s Disease. CNS Neurological Disordors-Drug Targets, 5, 355–361.
Spuch, C., Ortolano, S., & Navarro, C. (2012). New insights in the amyloid-beta interaction with mitochondria. Journal of Aging and Research, 2012, 324968.
Stein, B., Brady, H., Yang, M. X., Young, D. B., & Barbosa, M. S. (1996). Cloning and characterization of MEK6 a novel member of the mitogen activated protein kinase kinase cascade. Journal of Biological Chemistry, 271, 11427–11433.
Strooper, B. D., & Annaert, W. (2000). Proteolytic processing and cell biological functions of the amyloid precursor protein. Journal of Cell Sciences, 113, 1857–1870.
Sturchler, E., Feurstein, D., McDonald, P., & Duckett, D. (2010). Mechanism of oxidative stress-induced ASK1-catalyzed MKK6 phosphorylation. Biochemistry, 49, 4094–4102.
Su, B., Wang, X., Nunomura, A., Moreira, P. I., Lee, H., Perry, G., et al. (2008). Oxidative Stress Signaling in Alzheimer’s Disease. Current Alzheimer Research, 5, 525–532.
Tare, M., Modi, R. M., Nainaparampil, J. J., Puli, O. R., Bedi, S., Fernandez-Funez, P., et al. (2011). Activation of JNK signaling mediates amyloid-ss-dependent cell death. PLoS One, 6, e24361.
Tong, L., Thornton, P. L., Balazs, R., & Cotman, C. W. (2001). β-amyloid-(1–42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival is not compromised. Journal of Biological Chemistry, 276, 17301–17306.
Wang, H. Y., Lee, D. H., D’Andrea, M. R., Peterson, P. A., Shank, R. P., & Reitz, A. B. (2000). β-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. Journal Biological Chemistry, 275, 5626–5632.
Yankner, B. A., Dawes, L. R., Fisher, S., Villa-Komaroff, L., Oster-Granite, M. L., & Neve, R. L. (1989). Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science, 245, 417–420.
Zhu, X., Ogawa, O., Wang, Y., Perry, G., & Smith, M. A. (2003). JKK1, an upstream activator of JNK/SAPK, is activated in Alzheimer’s disease. Journal of Neurochemistry, 85, 87–93.
Acknowledgments
This work was supported in part by the Department of Science and Technology, Govt. of India, No: SR/SO/BB-09/2009 and by the University Grants Commission No F. 17-82/98(SA-I). Work was also supported by FIST (SR/FST/LSI-384/2008) and SAP (F.3-26/2011 (SAP-II) grants awarded to the department by the Department of Science & Technology, Govt. of India, and University Grants Commission, Govt. of India, respectively. We are grateful to Kaikobad Irani, Associate Professor at UPMC, USA, for providing ShRNA of p66shc and SS Andrabi, Fellow at Harvard University, USA, for providing WT p66shc and WT MKK6 plasmids.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rafia A. Baba and Hina F. Bhat have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Bashir, M., Parray, A.A., Baba, R.A. et al. β-Amyloid-evoked Apoptotic Cell Death is Mediated Through MKK6–p66shc Pathway. Neuromol Med 16, 137–149 (2014). https://doi.org/10.1007/s12017-013-8268-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8268-4