Abstract
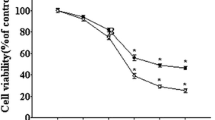
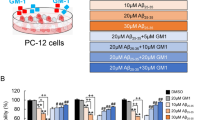
γ-Enolase acts as a neurotrophic-like factor promoting growth, differentiation, survival and regeneration of neurons. It is shown in this study to exert a protective effect against amyloid-β-peptide (Aβ)-induced neurotoxicity in rat pheochromocytoma PC12 cells. Aβ-induced toxicity was abolished in the presence of the active C-terminal peptide of γ-enolase (γ-Eno) as measured by cell viability, lactate dehydrogenase release, sub-G1 cell population, intracellular reactive oxygen species, mitochondrial functions and apoptotic morphology. γ-Eno caused downregulation of the pro-apoptotic protein Bax and upregulation of the anti-apoptotic protein Bcl-2, as well as reduced caspase-3 activation. Exposure to Aβ increased surface expression of p75 neurotrophin receptor (p75NTR), and the increase was abolished in the presence of γ-Eno peptide. Further, pretreatment with γ-Eno suppressed the activation of mitogen-activated protein kinases p38 and Jun-N-terminal kinase, which are p75NTR downstream effectors in apoptotic signaling. Moreover, Aβ triggered γ-enolase co-immunoprecipitation with p75NTR as well as their strong association in the perimembrane region as shown by confocal microscopy, which further supports the interaction between these two proteins in cells insulted by Aβ peptide. Our results indicate the possible use of γ-enolase C-terminal peptide for treating or preventing Alzheimer’s disease.








Similar content being viewed by others
References
Aloyz, R. S., Bamji, S. X., Pozniak, C. D., Toma, J. G., Atwal, J., Kaplan, D. R., et al. (1998). p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. Journal of Cell Biology, 143(6), 1691–1703.
Barker, P. A. (2004). p75NTR is positively promiscuous: Novel partners and new insights. Neuron, 42(4), 529–533.
Boldogh, I., & Kruzel, M. L. (2008). Colostrinin: An oxidative stress modulator for prevention and treatment of age-related disorders. Journal of Alzheimer’s Disease, 13(3), 303–321.
Braak, E., Griffing, K., Arai, K., Bohl, J., Bratzke, H., & Braak, H. (1999). Neuropathology of Alzheimer’s disease: What is new since A. Alzheimer? European Archives of Psychiatry and Clinical Neuroscience, 249, 14–22.
Butterfield, D. A., & Lange, M. L. (2009). Multifunctional roles of enolase in Alzheimer’s disease brain: Beyond altered glucose metabolism. Journal of Neurochemistry, 111, 915–933.
Cecchi, C., Fiorillo, C., Baglioni, S., Pensalfini, A., Bagnoli, S., Nacmias, B., et al. (2007). Increased susceptibility to amyloid toxicity in familial Alzheimer’s fibroblasts. Neurobiology of Aging, 28(6), 863–876.
Chakravarthy, B., Gaudet, C., Ménard, M., Atkinson, T., Brown, L., Laferla, F. M., et al. (2010). Amyloid-beta peptides stimulate the expression of the p75(NTR) neurotrophin receptor in SHSY5Y human neuroblastoma cells and AD transgenic mice. Journal of Alzheimer’s Disease, 19(3), 915–925.
Costantini, C., Rossi, F., Formaggio, E., Bernardoni, R., Cecconi, D., & Della-Bianca, V. (2005). Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in beta-amyloid peptide-dependent cell death. Journal of Molecular Neuroscience, 25(2), 141–156.
Dechant, G., & Barde, Y. A. (2002). The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nature Neuroscience, 5(11), 1131–1136.
Diarra, A., Geetha, T., Potter, P., & Babu, J. R. (2009). Signaling of the neurotrophin receptor p75 in relation to Alzheimer’s disease. Biochemical and Biophysical Research Communications, 390(3), 352–356.
Fletcher, L., Rider, C. C., & Taylor, C. B. (1976). Enolase isoenzymes: III. Chromatography and immunological characteristic of rat brain enolase. Biochimica et Biophysica Acta, 452, 245–252.
Forloni, G., Chiesa, R., Smiroldo, S., Verga, L., Salmona, M., Tagliavini, F., et al. (1993). Apoptosis mediated neurotoxicity induced by chronic application of beta amyloid fragment 25-35. NeuroReport, 4(5), 523–526.
Frasca, G., Chiechio, S., Vancheri, C., Nicoletti, F., Copani, A., & Angela Sortino, M. (2004). Beta-amyloid-activated cell cycle in SH-SY5Y neuroblastoma cells: Correlation with the MAP kinase pathway. Journal of Molecular Neuroscience, 22(3), 231–236.
Fukui, K., Takatsu, H., Shinkai, T., Suzuki, S., Abe, K., & Urano, S. (2005). Appearance of amyloid beta-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. Journal of Alzheimer’s Disease, 8(3), 299–309.
Gorman, A. M., McGowan, A., O’Neill, C., & Cotter, T. (1996). Oxidative stress and apoptosis in neurodegeneration. Journal of the Neurological Sciences, 139, 45–52.
Green, D. R., & Reed, J. C. (1998). Mitochondria and apoptosis. Science, 281(5381), 1309–1312.
Gross, A., McDonnell, J. M., & Korsmeyer, S. J. (1999). BCL-2 family members and the mitochondria in apoptosis. Genes & Development, 13(15), 1899–1911.
Hafner, A., Glavan, G., Obermajer, N., Zivin, M., Schliebs, R., & Kos, J. (2013). Neuroprotective role of γ-enolase in microglia in a mouse model of Alzheimer’s disease is regulated by cathepsin X. Aging Cell,. doi:10.1111/acel.12093.
Hafner, A., Obermajer, N., & Kos, J. (2010). γ1-Syntrophin mediates trafficking of γ-enolase towards the plasma membrane and enhances its neurotrophic activity. Neurosignals, 18, 246–258.
Hafner, A., Obermajer, N., & Kos, J. (2012). γ-enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of PI 3-K/Akt and MAPK/ERK signaling pathways. The Biochemical Journal, 443(2), 439–450.
Hardy, J., & Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science, 297(5580), 353–356.
Hattori, T., Ohsawa, K., Mizuno, Y., Kato, K., & Kohsaka, S. (1994). Synthetic peptide corresponding to 30 amino acids of the C-terminal of neuron-specific enolase promotes survival of neocortical neurons in culture. Biochemical and Biophysical Research Communications, 202, 25–30.
Hattori, T., Takei, N., Mizuno, Y., Kato, K., & Kohsaka, S. (1995). Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neuroscience Research, 21, 191–198.
Hensley, K., Carney, J. M., Mattson, M. P., Aksenova, M., Harris, M., Wu, J. F., et al. (1994). A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: Relevance to Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 91(8), 3270–3274.
Hu, X. Y., Zhang, H. Y., Qin, S., Xu, H., Swaab, D. F., & Zhou, J. N. (2002). Increased p75(NTR) expression in hippocampal neurons containing hyperphosphorylated tau in Alzheimer patients. Experimental Neurology, 178(1), 104–111.
Iversen, L. L., Mortishire-Smith, R. J., Pollack, S. J., & Shearman, M. S. (1995). The toxicity in vitro of beta-amyloid protein. The Biochemical Journal, 311, 1–16.
Junttila, M. R., Li, S. P., & Westermarck, J. (2008). Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. Federation of American Societies for Experimental Biology Journal, 22(4), 954–965.
Kadowaki, H., Nishitoh, H., Urano, F., Sadamitsu, C., Matsuzawa, A., Takeda, K., et al. (2005). Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death and Differentiation, 12(1), 19–24.
LeVine, H, 3rd. (1999). Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods in Enzymology, 309, 274–284.
Li, R. C., Pouranfar, F., Lee, S. K., Morris, M. W., Wang, Y., & Gozal, D. (2008). Neuroglobin protects PC12 cells against beta-amyloid-induced cell injury. Neurobiology of Aging, 29(12), 1815–1822.
Liu, Y., Guyton, K. Z., Gorospe, M., Xu, Q., Lee, J. C., & Holbrook, N. J. (1996). Differential activation of ERK, JNK/SAPK and P38/CSBP/RK map kinase family members during the cellular response to arsenite. Free Radical Biology & Medicine, 21(6), 771–781.
Longo, F. M., & Massa, S. M. (2004). Neurotrophin-based strategies for neuroprotection. Journal of Alzheimer’s Disease, 6(6 Suppl), S13–S17.
Loo, D. T., Copani, A., Pike, C. J., Whittemore, E. R., Walencewicz, A. J., & Cotman, C. W. (1993). Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proceedings of the National Academy of Sciences of the United States of America, 90(17), 7951–7955.
Lou, H., Fan, P., Perez, R. G., & Lou, H. (2011). Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorganic & Medicinal Chemistry, 19(13), 4021–4027.
Murn, J., Urleb, U., & Mlinaric-Rascan, I. (2004). Internucleosomal DNA cleavage in apoptotic WEHI 231 cells is mediated by a chymotrypsin-like protease. Genes to Cells, 9, 1103–1111.
Nagahara, A. H., Merrill, D. A., Coppola, G., Tsukada, S., Schroeder, B. E., Shaked, G. M., et al. (2009). Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nature Medicine, 15(3), 331–337.
Nagtegaal, I. D., Lakke, E. A., & Marani, E. (1998). Trophic and tropic factors in the development of the central nervous system. Archives of Physiology and Biochemistry, 106, 161–202.
Nykjaer, A., Lee, R., Teng, K. K., Jansen, P., Madsen, P., Nielsen, M. S., et al. (2004). Sortilin is essential for proNGF-induced neuronal cell death. Nature, 427(6977), 843–848.
Oh, Y., Kim, E. Y., Kim, Y., Jin, J., Jin, B. K., Jahng, G. H., et al. (2011). Neuroprotective effects of overexpressed cyclophilin B against Aβ-induced neurotoxicity in PC12 cells. Free Radical Biology & Medicine, 51(4), 905–920.
Pearn, M. L., Hu, Y., Niesmanm, I. R., Patel, H. H., Drummond, J. C., Roth, D. M., et al. (2012). Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology, 116(2), 352–361.
Pike, C. J., Walencewicz-Wasserman, A. J., Kosmoski, J., Cribbs, D. H., Glabe, C. G., & Cotman, C. W. (1995). Structure-activity analyses of beta-amyloid peptides: Contributions of the beta 25-35 region to aggregation and neurotoxicity. Journal of Neurochemistry, 64(1), 253–265.
Rabizadeh, S., Bitler, C. M., Butcher, L. L., & Bredesen, D. E. (1994). Expression of the low-affinity nerve growth factor receptor enhances beta-amyloid peptide toxicity. Proceedings of the National Academy of Sciences of the United States of America, 91(22), 10703–10706.
Rider, C. C., & Taylor, C. B. (1974). Enolase isoenzymes in rat tissues. Biochimica et Biophysica Acta, 365, 285–300.
Schmechel, D. E., Marangos, P. J., Martin, B. M., Winfield, S., Burkhart, D. S., Roses, A. D., et al. (1987). Localization of neuron-specific enolase (NSE) mRNA in human brain. Neuroscience Letters, 76, 233–238.
Schroeter, H., Boyd, C., Spencer, J. P., Williams, R. J., Cadenas, E., & Rice-Evans, C. (2002). MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiology of Aging, 23(5), 861–880.
Selkoe, D. J. (2000). Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Annals of the New York Academy of Sciences, 924, 17–25.
Shimohama, S. (2000). Apoptosis in Alzheimer’s disease—an update. Apoptosis, 5(1), 9–16.
Stadelmann, C., Deckwerth, T. L., Srinivasan, A., Bancher, C., Brück, W., Jellinger, K., et al. (1999). Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. American Journal of Pathology, 155(5), 1459–1466.
Takei, N., Kondo, J., Nagaike, K., Ohsawa, K., Kato, K., & Kohsaka, S. (1991). Neuronal survival factor from bovine brain is identical to neuron-specific enolase. Journal of Neurochemistry, 57, 1178–1184.
Tamagno, E., Parola, M., Guglielmotto, M., Santoro, G., Bardini, P., Marra, L., et al. (2003). Multiple signaling events in amyloid beta-induced, oxidative stress-dependent neuronal apoptosis. Free Radical Biology & Medicine, 35(1), 45–58.
Tuszynski, M. H. (2007). Nerve growth factor gene therapy in Alzheimer disease. Alzheimer Disease and Associated Disorders, 21(2), 179–189.
Vander Heiden, M. G., & Thompson, C. B. (1999). Bcl-2 proteins: Regulators of apoptosis or of mitochondrial homeostasis? Nature Cell Biology, 1, 209–216.
Wilquet, V., & De Strooper, B. (2004). Amyloid-beta precursor protein processing in neurodegeneration. Current Opinion in Neurobiology, 4(5), 582–588.
Xia, Z., Dickens, M., Raingeaud, J., Davis, R. J., & Greenberg, M. E. (1995). Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 270(5240), 1326–1331.
Xian, Y. F., Lin, Z. X., Mao, Q. Q., Ip, S. P., Su, Z. R., & Lai, X. P. (2012). Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cellular and Molecular Neurobiology, 32(3), 353–360.
Yaar, M., Zhai, S., Pilch, P. F., Doyle, S. M., Eisenhauer, P. B., et al. (1997). Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease. The Journal of Clinical Investigation, 100(9), 2333–2340.
Yakovlev, A. G., & Faden, A. I. (2001). Caspase-dependent apoptotic pathways in CNS injury. Molecular Neurobiology, 24(1–3), 131–144.
Yu, M. S., Suen, K. C., Kwok, N. S., So, K. F., Hugon, J., & Chang, R. C. (2006). Beta-amyloid peptides induces neuronal apoptosis via a mechanism independent of unfolded protein responses. Apoptosis, 11(5), 687–700.
Zhang, Y., Hong, Y., Bounhar, Y., Blacker, M., Roucou, X., Tounekti, O., et al. (2003). p75 neurotrophin receptor protects primary cultures of human neurons against extracellular amyloid beta peptide cytotoxicity. Journal of Neuroscience, 23(19), 7385–7394.
Zhang, L., Yu, H., Zhao, X., Lin, X., Tan, C., Cao, G., et al. (2010). Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochemistry International, 57(5), 547–555.
Zhu, X., Raina, A. K., Lee, H. G., Casadesus, G., Smith, M. A., & Perry, G. (2004). Oxidative stress signalling in Alzheimer’s disease. Brain Research, 1000(1–2), 32–39.
Acknowledgments
We acknowledge Professor Roger Pain for critical review of the paper before submission. This study was supported by the Research Agency of Republic of Slovenia (grant numbers P4-0127 and J4-4123 to JK).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12017_2013_8247_MOESM1_ESM.tif
Fibril formation of amyloid-β peptides Aβ25-35 and Aβ1-42. Aβ were incubated at 37˚C for 5 days and then fibril formation was determined using the thioflavine T assay. Graph represents fluorescence thioflavine T signal of Aβ25-35 (30 μM) and Aβ1-42Aβ (5 μM) in 0.215 M sodium phosphate buffer (pH 8.0) for 5 days. The values are means ± s.d. of three independent assays. *P < 0.01 (TIFF 2191 kb)
12017_2013_8247_MOESM2_ESM.tif
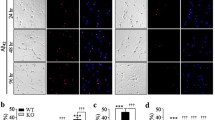
Effect of Pep5 and γ-Eno peptide on Aβ25-35-induced γ-enolase and p75NTR association in PC12 cells. a, b Representative images of co-localization of p75NTR (green fluorescence) and γ-enolase (red fluorescence) in PC12 cells pretreated with p75NTR inhibitor Pep5 (5 µM) (a) or γ-Eno peptide(100 nM) (b) and further treated with Aβ25-35 (30 µM) for 3 h, as analyzed by confocal microscopy. Scale bar = 10 µm (TIFF 6311 kb)
Rights and permissions
About this article
Cite this article
Pišlar, A.H., Kos, J. C-Terminal Peptide of γ-Enolase Impairs Amyloid-β-Induced Apoptosis Through p75NTR Signaling. Neuromol Med 15, 623–635 (2013). https://doi.org/10.1007/s12017-013-8247-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8247-9