Abstract
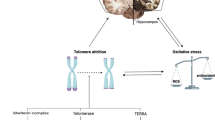
Telomeres, at the ends of chromosomes and strands of genetic material, become shorter as cells divide in the process of aging. Telomere length has been considered as a biological marker of age. Telomere length shortening has also been evidenced as the causable role in age-related neurodegenerative diseases, including Alzheimer’s disease (AD). It has been demonstrated that telomere shortening has been associated with cognitive impairment, amyloid pathology and hyper-phosphorylation of tau in AD and plays an important role in the pathogenesis of AD via the mechanism of oxidative stress and inflammation. However, it seems that there is no relationship between telomere shortening and AD. Therefore, it is essential for further clarification of telomere-related pathogenesis in AD.

Similar content being viewed by others
Abbreviations
- Aβ :
-
Beta-amyloid
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- BACE:
-
Beta-site APP-cleaving enzyme
- BBB:
-
Blood brain barrier
- BFB:
-
Breakage/fusion/bridge cycle
- CADASIL:
-
Cerebral autosomal dominant arteriopathy and leukoencephalopathy
- COPD:
-
Chronic obstructive pulmonary disease
- Cox-2:
-
Cyclooxygenase 2
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- eNOS:
-
Endothelial nitric oxide synthase
- ICAM-1:
-
Inter-cellular adhesion molecule 1
- ECM:
-
Extracellular matrix
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- HD:
-
Huntington’s disease
- IFN:
-
Interferon
- LIF:
-
Leukemia inhibitory factor
- IL:
-
Interleukin
- IRAK:
-
Interleukin-1 receptor-associated kinase
- IRE:
-
Iron-responsive element
- LRP-1:
-
Low-density lipoprotein receptor-related protein 1
- MAPK:
-
Mitogen-activated protein kinase
- MCI:
-
Mild cognitive impairment
- MCP-1:
-
Macrophage chemoattractant protein 1
- MDS:
-
Myelodysplastic syndromes
- MMPs:
-
Matrix metalloproteinases
- MMSE:
-
Mini Mental Status Examination
- NF-κB:
-
Nuclear factor-kappa B
- NFT:
-
Neurofibrillary tangles
- NO:
-
Nitric oxide
- OSA:
-
Obstructive sleep apnea
- PD:
-
Parkinson’s disease
- RAGE:
-
Receptor for advanced glycation end products
- ROS:
-
Reactive oxygen species
- SAS:
-
Sleep apnea syndrome
- TGF-β :
-
Transforming growth factor beta
- TNF-α :
-
Tumor necrosis factor-alpha
- TNFR:
-
Tumor necrosis factor receptor
- TRADD:
-
TNF receptor-associated death domain
- 8-oxodG:
-
8-Oxo-2′-deoxyguanosine
References
Adaikalakoteswari, A., Balasubramanyam, M., & Mohan, V. (2005). Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabetic Medicine, 22, 1151–1156.
Adaikalakoteswari, A., Balasubramanyam, M., Ravikumar, R., Deepa, R., & Mohan, V. (2007). Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis, 195, 83–89.
Agostinho, P., Cunha, R. A., & Oliveira, C. (2010). Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Current Pharmaceutical Design, 16, 2766–2778.
Aguade, M., Meyers, W., Long, A. D., & Langley, C. H. (1994). Single-strand conformation polymorphism analysis coupled with stratified DNA sequencing reveals reduced sequence variation in the su(s) and su(wa) regions of the Drosophila melanogaster X chromosome. Proceedings of the National Academy of Sciences of the United States of America, 91, 4658–4662.
Aikata, H., Takaishi, H., Kawakami, Y., Takahashi, S., Kitamoto, M., Nakanishi, T., et al. (2000). Telomere reduction in human liver tissues with age and chronic inflammation. Experimental Cell Research, 256, 578–582.
Al-Attas, O. S., Al-Daghri, N., Bamakhramah, A., Shaun Sabico, S., McTernan, P., & Huang, T. T. (2010). Telomere length in relation to insulin resistance, inflammation and obesity among Arab youth. Acta Paediatrica, 99, 896–899.
Aliev, G. (2011). Oxidative stress induced-metabolic imbalance, mitochondrial failure, and cellular hypoperfusion as primary pathogenetic factors for the development of Alzheimer disease which can be used as a alternate and successful drug treatment strategy: past, present and future. CNS & Neurological Disorders: Drug Targets, 10, 147–148.
Allsopp, R. C., Chang, E., Kashefi-Aazam, M., Rogaev, E. I., Piatyszek, M. A., Shay, J. W., et al. (1995). Telomere shortening is associated with cell division in vitro and in vivo. Experimental Cell Research, 220, 194–200.
Aluise, C. D., Robinson, R. A., Beckett, T. L., Murphy, M. P., Cai, J., Pierce, W. M., et al. (2010). Preclinical Alzheimer disease: Brain oxidative stress, Abeta peptide and proteomics. Neurobiology of Diseases, 39, 221–228.
Amsellem, V., Gary-Bobo, G., Marcos, E., Maitre, B., Chaar, V., Validire, P., et al. (2011). Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 184(12), 1358–1366.
Anchisi, D., Borroni, B., Franceschi, M., Kerrouche, N., Kalbe, E., Beuthien-Beumann, B., et al. (2005). Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer disease. Archives of Neurology, 62, 1728–1733.
Andersen, J. K. (2004). Oxidative stress in neurodegeneration: Cause or consequence? Natural Medicine, 10(Suppl), S18–S25.
Andrews, N. P., Fujii, H., Goronzy, J. J., & Weyand, C. M. (2010). Telomeres and immunological diseases of aging. Gerontology, 56, 390–403.
Ansari, M. A., & Scheff, S. W. (2010). Oxidative stress in the progression of Alzheimer disease in the frontal cortex. Journal of Neuropathology and Experimental Neurology, 69, 155–167.
Apelt, J., Bigl, M., Wunderlich, P., & Schliebs, R. (2004). Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. International Journal of Developmental Neuroscience, 22, 475–484.
Aukrust, P., Berge, R. K., Ueland, T., Aaser, E., Damas, J. K., Wikeby, L., et al. (2001). Interaction between chemokines and oxidative stress: Possible pathogenic role in acute coronary syndromes. Journal of the American College of Cardiology, 37, 485–491.
Aviv, A. (2004). Telomeres and human aging: facts and fibs. Science of Aging Knowledge Environment, 2004(51), pe43.
Aviv, A., Valdes, A., Gardner, J. P., Swaminathan, R., Kimura, M., & Spector, T. D. (2006). Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. Journal of Clinical Endocrinology and Metabolism, 91, 635–640.
Ayasolla, K., Khan, M., Singh, A. K., & Singh, I. (2004). Inflammatory mediator and beta-amyloid (25–35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radical Biology and Medicine, 37, 325–338.
Babizhayev, M. A., Savel’yeva, E. L., Moskvina S. N., & Yegorov Y. E. (2011a). Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. American Journal of Therapeutics, 18(6), e209–e226.
Babizhayev, M. A., Vishnyakova, K. S., & Yegorov, Y. E. (2011b). Telomere-dependent senescent phenotype of lens epithelial cells as a biological marker of aging and cataractogenesis: The role of oxidative stress intensity and specific mechanism of phospholipid hydroperoxide toxicity in lens and aqueous. Fundamental & Clinical Pharmacology, 25, 139–162.
Babizhayev, M. A., & Yegorov, Y. E. (2011). Smoking and health: Association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fundamental & Clinical Pharmacology, 25, 425–442.
Bar-Or, D., Thomas, G. W., Rael, L. T., Lau, E. P., & Winkler, J. V. (2001). Asp-Ala-His-Lys (DAHK) inhibits copper-induced oxidative DNA double strand breaks and telomere shortening. Biochemical and Biophysical Research Communications, 282, 356–360.
Bates, G. P., MacDonald, M. E., Baxendale, S., Sedlacek, Z., Youngman, S., Romano, D., et al. (1990). A yeast artificial chromosome telomere clone spanning a possible location of the Huntington disease gene. American Journal of Human Genetics, 46, 762–775.
Bauer, M. E., Jeckel, C. M., & Luz, C. (2009). The role of stress factors during aging of the immune system. Annals of the New York Academy of Sciences, 1153, 139–152.
Baumer, Y., Scholz, B., Ivanov, S., & Schlosshauer, B. (2011). Telomerase-based immortalization modifies the angiogenic/inflammatory responses of human coronary artery endothelial cells. Experimental Biology and Medicine (Maywood), 236, 692–700.
Bayer, T. A., Schafer, S., Breyhan, H., Wirths, O., Treiber, C., & Multhaup, G. (2006). A vicious circle: role of oxidative stress, intraneuronal Abeta and Cu in Alzheimer’s disease. Clinical Neuropathology, 25, 163–171.
Bayne, S., Li, H., Jones, M. E., Pinto, A. R., van Sinderen, M., Drummond, A., et al. (2011). Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell, 2, 333–346.
Bekaert, S., De Meyer, T., Rietzschel, E. R., De Buyzere, M. L., De Bacquer, D., Langlois, M., et al. (2007). Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell, 6, 639–647.
Bennett, D. A., Schneider, J. A., Bienias, J. L., Evans, D. A., & Wilson, R. S. (2005). Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology, 64, 834–841.
Bergamaschini, L., Donarini, C., Gobbo, G., Parnetti, L., & Gallai, V. (2001). Activation of complement and contact system in Alzheimer’s disease. Mechanisms of Ageing and Development, 122, 1971–1983.
Berkenbosch, F., Biewenga, J., Brouns, M., Rozemuller, J. M., Strijbos, P., & van Dam, A. M. (1992). Cytokines and inflammatory proteins in Alzheimer’s disease. Research in Immunology, 143, 657–663.
Bhattacharjee, R. N., Banerjee, B., Akira, S., & Hande, M. P. (2010). Telomere-mediated chromosomal instability triggers TLR4 induced inflammation and death in mice. PLoS ONE, 5, e11873.
Bonda, D. J., Wang, X., Perry, G., Nunomura, A., Tabaton, M., Zhu, X., et al. (2010). Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology, 59, 290–294.
Boukamp, P., & Mirancea, N. (2007). Telomeres rather than telomerase a key target for anti-cancer therapy? Experimental Dermatology, 16, 71–79.
Bracco, L., Gallato, R., Grigoletto, F., Lippi, A., Lepore, V., Bino, G., et al. (1994). Factors affecting course and survival in Alzheimer’s disease. A 9-year longitudinal study. Archives of Neurology, 51, 1213–1219.
Brandt, C. A., Kierkegaard, O., Hindkjaer, J., Jensen, P. K., Pedersen, S., & Therkelsen, A. J. (1993). Ring chromosome 20 with loss of telomeric sequences detected by multicolour PRINS. Clinical Genetics, 44, 26–31.
Broberg, K., Bjork, J., Paulsson, K., Hoglund, M., & Albin, M. (2005). Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis, 26, 1263–1271.
Broccoli, D., Godley, L. A., Donehower, L. A., Varmus, H. E., & de Lange, T. (1996). Telomerase activation in mouse mammary tumors: Lack of detectable telomere shortening and evidence for regulation of telomerase RNA with cell proliferation. Molecular and Cellular Biology, 16, 3765–3772.
Brown, P. J., Devanand, D. P., Liu, X., & Caccappolo, E. (2011). Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Archives of General Psychiatry, 68, 617–626.
Brown, W. R., MacKinnon, P. J., Villasante, A., Spurr, N., Buckle, V. J., & Dobson, M. J. (1990). Structure and polymorphism of human telomere-associated DNA. Cell, 63, 119–132.
Bunout, D., & Cambiazo, V. (1999). Nutrition and aging. Revista Medica de Chile, 127, 82–88.
Butterfield, D. A. (2011). Oxidative stress in Alzheimer disease: synergy between the Butterfield and Markesbery laboratories. Neuromolecular Medicine, 13, 19–22.
Butterfield, D. A., & Boyd-Kimball, D. (2004). Amyloid beta-peptide(1–42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathology, 14, 426–432.
Butterfield, D. A., Galvan, V., Lange, M. B., Tang, H., Sowell, R. A., Spilman, P., et al. (2010). In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radical Biology and Medicine, 48, 136–144.
Butterfield, D. A., Griffin, S., Munch, G., & Pasinetti, G. M. (2002). Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. Journal of Alzheimer’s Disease, 4, 193–201.
Butterfield, D. A., & Lauderback, C. M. (2002). Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radical Biology and Medicine, 32, 1050–1060.
Butterfield, D. A., Reed, T., Newman, S. F., & Sultana, R. (2007). Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radical Biology and Medicine, 43, 658–677.
Cai, Z., Zhao, Y., Yao, S., & Bin Zhao, B. (2011). Increases in beta-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-kappaB pathway activation. Pharmacological Reports, 63, 381–391.
Calissano, P., Matrone, C., & Amadoro, G. (2009). Apoptosis and in vitro Alzheimer disease neuronal models. Communicative & Integrative Biology, 2, 163–169.
Cameron, B., & Landreth, G. E. (2010). Inflammation, microglia, and Alzheimer’s disease. Neurobiology of Diseases, 37, 503–509.
Candore, G., Bulati, M., Caruso, C., Castiglia, L., Colonna-Romano, G., Di Bona, D., et al. (2010). Inflammation, cytokines, immune response, apolipoprotein E, cholesterol, and oxidative stress in Alzheimer disease: Therapeutic implications. Rejuvenation Research, 13, 301–313.
Canu, N., & Calissano, P. (2003). In vitro cultured neurons for molecular studies correlating apoptosis with events related to Alzheimer disease. Cerebellum, 2, 270–278.
Cattan, V., Mercier, N., Gardner, J. P., Regnault, V., Labat, C., Maki-Jouppila, J., et al. (2008). Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radical Biology and Medicine, 44, 1592–1598.
Chan, R., Woo, J., Suen, E., Leung, J., & Tang, N. (2010). Chinese tea consumption is associated with longer telomere length in elderly Chinese men. British Journal of Nutrition, 103, 107–113.
Chawla, R., Redon, S., Raftopoulou, C., Wischnewski, H., Gagos, S., & Azzalin, C. M. (2011). Human UPF1 interacts with TPP1 and telomerase and sustains telomere leading-strand replication. The European Molecular Biology Organization Journal, 30(19), 4047–4058.
Chen, W., Gardner, J. P., Kimura, M., Brimacombe, M., Cao, X., Srinivasan, S. R., et al. (2009). Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis, 205, 620–625.
Chen, W., Kimura, M., Kim, S., Cao, X., Srinivasan, S. R., Berenson, G. S., et al. (2011). Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: Age-dependent telomere shortening is the rule. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 66, 312–319.
Cheng, S. V., Martin, G. R., Nadeau, J. H., Haines, J. L., Bucan, M., Kozak, C. A., et al. (1989). Synteny on mouse chromosome 5 of homologs for human DNA loci linked to the Huntington disease gene. Genomics, 4, 419–426.
Cheung, A. L., & Deng, W. (2008). Telomere dysfunction, genome instability and cancer. Frontiers in Bioscience, 13, 2075–2090.
Christen, Y. (2000). Oxidative stress and Alzheimer disease. American Journal of Clinical Nutrition, 71, 621S–629S.
Cipriano, C., Tesei, S., Malavolta, M., Giacconi, R., Muti, E., Costarelli, L., et al. (2009). Accumulation of cells with short telomeres is associated with impaired zinc homeostasis and inflammation in old hypertensive participants. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 64, 745–751.
Clark, T. A., Lee, H. P., Rolston, R. K., Zhu, X., Marlatt, M. W., Castellani, R. J., et al. (2010). Oxidative stress and its implications for future treatments and management of Alzheimer disease. International Journal of Biomedical Sciences, 6, 225–227.
Collerton, J., Martin-Ruiz, C., Kenny, A., Barrass, K., von Zglinicki, T., Kirkwood, T., et al. (2007). Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ study. European Heart Journal, 28, 172–176.
Corkin, S., Growdon, J. H., & Rasmussen, S. L. (1983). Parental age as a risk factor in Alzheimer’s disease. Annals of Neurology, 13, 674–676.
Costenbader, K. H., Prescott, J., Zee, R. Y., & De Vivo, I. (2011). Immunosenescence and rheumatoid arthritis: Does telomere shortening predict impending disease? Autoimmunity Reviews, 10, 569–573.
Craft, S., Baker, L. D., Montine, T. J., Minoshima, S., Watson, G. S., Claxton, A., et al. (2012). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Archives of neurology, 69(1), 29–38.
Crews, L., & Masliah, E. (2010). Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Human Molecular Genetics, 19, R12–R20.
Crossen, P. E., Tully, S. M., Benjes, S. M., Hollings, P. E., Beard, M. E., Nimmo, J. C., et al. (1993). Oligoclonal B-cell leukemia characterized by spontaneous cell division and telomere association. Genes, Chromosomes and Cancer, 8, 49–59.
Cuschieri, J., & Maier, R. V. (2007). Oxidative stress, lipid rafts, and macrophage reprogramming. Antioxidants & Redox Signaling, 9, 1485–1497.
Damjanovic, A. K., Yang, Y., Glaser, R., Kiecolt-Glaser, J. K., Nguyen, H., Laskowski, B., et al. (2007). Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. Journal of Immunology, 179, 4249–4254.
de Jesus, B. B., Schneeberger, K., Vera, E., Tejera, A., Harley, C. B., & Blasco, M. A. (2011). The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell, 10, 604–621.
De Meyer, T., Rietzschel, E. R., De Buyzere, M. L., Van Criekinge, W., & Bekaert, S. (2008). Studying telomeres in a longitudinal population based study. Frontiers in Bioscience, 13, 2960–2970.
Deane, R., Wu, Z., & Zlokovic, B. V. (2004). RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke, 35, 2628–2631.
Demissie, S., Levy, D., Benjamin, E. J., Cupples, L. A., Gardner, J. P., Herbert, A., et al. (2006). Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell, 5, 325–330.
den Elzen, W. P., & Gussekloo, J. (2011). Anaemia in older persons. Netherlands Journal of Medicine, 69, 260–267.
DePinho, R. A., & Wong, K. K. (2003). The age of cancer: Telomeres, checkpoints, and longevity. Journal of Clinical Investigation, 111, S9–S14.
Devore, E. E., Prescott, J., De Vivo, I., & Grodstein, F. (2011). Relative telomere length and cognitive decline in the Nurses’ Health Study. Neuroscience Letters, 492, 15–18.
Diaz, V. A., Mainous, A. G., 3rd, Everett, C. J., Schoepf, U. J., Codd, V., & Samani, N. J. (2010a). Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. American Journal of Cardiology, 106, 659–663.
Diaz, V. A., Mainous, A. G., Player, M. S., & Everett, C. J. (2010b). Telomere length and adiposity in a racially diverse sample. International Journal of Obesity (London), 34, 261–265.
D’Introno, A., Solfrizzi, V., Colacicco, A. M., Capurso, C., Amodio, M., Todarello, O., et al. (2006). Current knowledge of chromosome 12 susceptibility genes for late-onset Alzheimer’s disease. Neurobiology of Aging, 27, 1537–1553.
Doggett, N. A., Cheng, J. F., Smith, C. L., & Cantor, C. R. (1989). The Huntington disease locus is most likely within 325 kilobases of the chromosome 4p telomere. Proceedings of the National Academy of Sciences of the United States of America, 86, 10011–10014.
Donahue, J. E., Flaherty, S. L., Johanson, C. E., Duncan, J. A., 3rd, Silverberg, G. D., Miller, M. C., et al. (2006). RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathologica, 112, 405–415.
Eerola, J., Kananen, L., Manninen, K., Hellstrom, O., Tienari, P. J., & Hovatta, I. (2010). No evidence for shorter leukocyte telomere length in Parkinson’s disease patients. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 65, 1181–1184.
Efendic, E., Lindholm, B., Bergstrom, J., & Stenvinkel, P. (1999). Strong connection between malnutrition, inflammation and arteriosclerosis. Improved treatment of renal failure if underlying factors are attacked. Lakartidningen, 96, 4538–4542.
Epel, E. S., Merkin, S. S., Cawthon, R., Blackburn, E. H., Adler, N. E., Pletcher, M. J., et al. (2009). The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany, NY), 1, 81–88.
Farrag, W., Eid, M., El-Shazly, S., & Abdallah, M. (2011). Angiotensin II type 1 receptor gene polymorphism and telomere shortening in essential hypertension. Molecular and Cellular Biochemistry, 351, 13–18.
Feng, X., Wang, L., & Li, Y. (2011). Change of telomere length in angiotensin II-induced human glomerular mesangial cell senescence and the protective role of losartan. Molecular Medicine Reports, 4, 255–260.
Ferretti, M. T., Bruno, M. A., Ducatenzeiler, A., Klein, W. L., & Cuello, A. C. (2012). Intracellular Abeta-oligomers and early inflammation in a model of Alzheimer’s disease. Neurobiology of Aging, 33(7), 1329–1342.
Fiala, M., & Veerhuis, R. (2010). Biomarkers of inflammation and amyloid-beta phagocytosis in patients at risk of Alzheimer disease. Experimental Gerontology, 45, 57–63.
Finder, V. H. (2010). Alzheimer’s disease: A general introduction and pathomechanism. Journal of Alzheimer’s Disease, 22(Suppl 3), 5–19.
Fitzpatrick, A. L., Kronmal, R. A., Gardner, J. P., Psaty, B. M., Jenny, N. S., Tracy, R. P., et al. (2007). Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology, 165, 14–21.
Fitzpatrick, A. L., Kronmal, R. A., Kimura, M., Gardner, J. P., Psaty, B. M., Jenny, N. S., et al. (2011). Leukocyte telomere length and mortality in the Cardiovascular Health Study. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 66, 421–429.
Flanary, B. E., Sammons, N. W., Nguyen, C., Walker, D., & Streit, W. J. (2007). Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Research, 10, 61–74.
Flirski, M., & Sobow, T. (2005). Biochemical markers and risk factors of Alzheimer’s disease. Current Alzheimer Research, 2, 47–64.
Fujii, H., Shao, L., Colmegna, I., Goronzy, J. J., & Weyand, C. M. (2009). Telomerase insufficiency in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America, 106, 4360–4365.
Furumoto, K., Inoue, E., Nagao, N., Hiyama, E., & Miwa, N. (1998). Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sciences, 63, 935–948.
Fyhrquist, F., Silventoinen, K., Saijonmaa, O., Kontula, K., Devereux, R. B., de Faire, U., et al. (2011). Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: The LIFE study. Journal of Human Hypertension, 25(12), 711–718.
Garcia-Cruz, D., Vasquez, A. I., Perez-Rulfo, D., Davalos, N. O., Penaloza, J., Garcia-Ortiz, J. E., et al. (2000). Ring-20-syndrome and loss of telomeric regions. Annales de Genetique, 43, 113–116.
Gaynutdinov, T. I., Brown, P., Neumann, R. D., & Panyutin, I. G. (2009). Duplex formation at the 5’ end affects the quadruplex conformation of the human telomeric repeat overhang in sodium but not in potassium. Biochemistry, 48, 11169–11177.
Gella, A., & Durany, N. (2009). Oxidative stress in Alzheimer disease. Cell Adhesion & Migration, 3, 88–93.
Georgin-Lavialle, S., Aouba, A., Mouthon, L., Londono-Vallejo, J. A., Lepelletier, Y., Gabet, A. S., et al. (2010). The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmunity Reviews, 9, 646–651.
Getliffe, K. M., Martin Ruiz, C., Passos, J. F., von Zglinicki, T., & Nwokolo, C. U. (2006). Extended lifespan and long telomeres in rectal fibroblasts from late-onset ulcerative colitis patients. European Journal of Gastroenterology and Hepatology, 18, 133–141.
Gibson, G. E., Park, L. C., Zhang, H., Sorbi, S., & Calingasan, N. Y. (1999). Oxidative stress and a key metabolic enzyme in Alzheimer brains, cultured cells, and an animal model of chronic oxidative deficits. Annals of the New York Academy of Sciences, 893, 79–94.
Gilley, D., & Blackburn, E. H. (1994). Lack of telomere shortening during senescence in Paramecium. Proceedings of the National Academy of Sciences of the United States of America, 91, 1955–1958.
Gilliam, T. C., Tanzi, R. E., Haines, J. L., Bonner, T. I., Faryniarz, A. G., Hobbs, W. J., et al. (1987). Localization of the Huntington’s disease gene to a small segment of chromosome 4 flanked by D4S10 and the telomere. Cell, 50, 565–571.
Gisselsson, D., Jonson, T., Petersen, A., Strombeck, B., Dal Cin, P., Hoglund, M., et al. (2001). Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proceedings of the National Academy of Sciences of the United States of America, 98, 12683–12688.
Golde, T. E. (2002). Inflammation takes on Alzheimer disease. Nature Medicine, 8, 936–938.
Grandin, N., & Charbonneau, M. (2008). Protection against chromosome degradation at the telomeres. Biochimie, 90, 41–59.
Grodstein, F., van Oijen, M., Irizarry, M. C., Rosas, H. D., Hyman, B. T., Growdon, J. H., et al. (2008). Shorter telomeres may mark early risk of dementia: Preliminary analysis of 62 participants from the nurses’ health study. PLoS ONE, 3, e1590.
Gu, B. W., Fan, J. M., Bessler, M., & Mason, P. J. (2011). Accelerated hematopoietic stem cell aging in a mouse model of dyskeratosis congenita responds to antioxidant treatment. Aging Cell, 10, 338–348.
Guan, J. Z., Guan, W. P., Maeda, T., & Makino, N. (2012). Effect of vitamin E administration on the elevated oxygen stress and the telomeric and subtelomeric status in Alzheimer’s disease. Gerontology, 58(1), 62–69.
Guan, J. Z., Maeda, T., Sugano, M., Oyama, J., Higuchi, Y., Suzuki, T., et al. (2008). A percentage analysis of the telomere length in Parkinson’s disease patients. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 63, 467–473.
Guerreiro, R. J., Santana, I., Bras, J. M., Santiago, B., Paiva, A., & Oliveira, C. (2007). Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neurodegenerative Diseases, 4, 406–412.
Gupta, N., Taneja, R., Pandey, A., Mukesh, M., Singh, H., & Gupta, S. C. (2007). Replicative senescence, telomere shortening and cell proliferation rate in Gaddi goat’s skin fibroblast cell line. Cell Biology International, 31, 1257–1264.
Gusella, J. F., Gilliam, T. C., Tanzi, R. E., MacDonald, M. E., Cheng, S. V., Wallace, M., et al. (1986). Molecular genetics of Huntington’s disease. Cold Spring Harbor Symposia on Quantitative Biology, 51(Pt 1), 359–364.
Halaschek-Wiener, J., Vulto, I., Fornika, D., Collins, J., Connors, J. M., Le, N. D., et al. (2008). Reduced telomere length variation in healthy oldest old. Mechanisms of Ageing and Development, 129, 638–641.
Hamet, P., & Tremblay, J. (2003). Genes of aging. Metabolism, 52, 5–9.
Harley, C. B., Liu, W., Blasco, M., Vera, E., Andrews, W. H., Briggs, L. A., et al. (2011). A natural product telomerase activator as part of a health maintenance program. Rejuvenation Research, 14, 45–56.
Harris, S. E., Deary, I. J., MacIntyre, A., Lamb, K. J., Radhakrishnan, K., Starr, J. M., et al. (2006). The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neuroscience Letters, 406, 260–264.
Harris, S. E., Martin-Ruiz, C., von Zglinicki, T., Starr, J. M., & Deary, I. J. (2012). Telomere length and aging biomarkers in 70-year-olds: The Lothian Birth Cohort 1936. Neurobiology of Aging, 33(7), 1486.e3–1486.e8.
Herbert, K. E., Mistry, Y., Hastings, R., Poolman, T., Niklason, L., & Williams, B. (2008). Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circulation Research, 102, 201–208.
Herring, A., Blome, M., Ambree, O., Sachser, N., Paulus, W., & Keyvani, K. (2010). Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathology, 20, 166–175.
Holmes, C., & Butchart, J. (2011). Systemic inflammation and Alzheimer’s disease. Biochemical Society Transactions, 39, 898–901.
Honig, L. S., Schupf, N., Lee, J. H., Tang, M. X., & Mayeux, R. (2006). Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Annals of Neurology, 60, 181–187.
Hosgood, H. D., 3rd, Cawthon, R., He, X., Chanock, S., & Lan, Q. (2009). Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer, 66, 157–161.
Hou, L., Savage, S. A., Blaser, M. J., Perez-Perez, G., Hoxha, M., Dioni, L., et al. (2009). Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiology, Biomarkers & Prevention, 18, 3103–3109.
Houben, J. M., Mercken, E. M., Ketelslegers, H. B., Bast, A., Wouters, E. F., Hageman, G. J., et al. (2009). Telomere shortening in chronic obstructive pulmonary disease. Respiratory Medicine, 103, 230–236.
Houben, J. M., Moonen, H. J., van Schooten, F. J., & Hageman, G. J. (2008). Telomere length assessment: Biomarker of chronic oxidative stress? Free Radical Biology and Medicine, 44, 235–246.
Huang, J., Okuka, M., McLean, M., Keefe, D. L., & Liu, L. (2010). Telomere susceptibility to cigarette smoke-induced oxidative damage and chromosomal instability of mouse embryos in vitro. Free Radical Biology and Medicine, 48, 1663–1676.
Hudson, G., Faini, D., Stutt, A., Eccles, M., Robinson, L., Burn, D. J., et al. (2011). No evidence of substantia nigra telomere shortening in Parkinson’s disease. Neurobiology of Aging, 32(11), 2107.e3–2107.e5.
Huerta, C., Alvarez, V., Mata, I. F., Coto, E., Ribacoba, R., Martinez, C., et al. (2004). Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer’s and Parkinson’s disease. Neuroscience Letters, 370, 151–154.
Ilmonen, P., Kotrschal, A., & Penn, D. J. (2008). Telomere attrition due to infection. PLoS ONE, 3, e2143.
Insel, K. C., Merkle, C. J., Hsiao, C. P., Vidrine, A. N., & Montgomery, D. W. (2012). Biomarkers for cognitive aging–part I: Telomere length, blood pressure and cognition among individuals with hypertension. Biological Research for Nursing, 14(2), 124–132.
Isokawa, O., Suda, T., Aoyagi, Y., Kawai, H., Yokota, T., Takahashi, T., et al. (1999). Reduction of telomeric repeats as a possible predictor for development of hepatocellular carcinoma: Convenient evaluation by slot-blot analysis. Hepatology, 30, 408–412.
Jain, D., & Cooper, J. P. (2010). Telomeric strategies: Means to an end. Annual Review of Genetics, 44, 243–269.
James, B. D., Boyle, P. A., Buchman, A. S., Barnes, L. L., & Bennett, D. A. (2011). Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. The American Journal of Geriatric Psychiatry, 19(11), 961–969.
Jaskelioff, M., Muller, F. L., Paik, J. H., Thomas, E., Jiang, S., Adams, A. C., et al. (2011). Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature, 469, 102–106.
Jenkins, E. C., Velinov, M. T., Ye, L., Gu, H., Li, S., Jenkins, E. C., Jr., et al. (2006). Telomere shortening in T lymphocytes of older individuals with Down syndrome and dementia. Neurobiology of Aging, 27, 941–945.
Jenkins, E. C., Ye, L., Gu, H., Ni, S. A., Duncan, C. J., Velinov, M., et al. (2008). Increased “absence” of telomeres may indicate Alzheimer’s disease/dementia status in older individuals with Down syndrome. Neuroscience Letters, 440, 340–343.
Jennings, B. J., Ozanne, S. E., & Hales, C. N. (2000). Nutrition, oxidative damage, telomere shortening, and cellular senescence: Individual or connected agents of aging? Molecular Genetics and Metabolism, 71, 32–42.
Jeynes, B., & Provias, J. (2008). Evidence for altered LRP/RAGE expression in Alzheimer lesion pathogenesis. Current Alzheimer Research, 5, 432–437.
Jia, J. P., Meng, R., Sun, Y. X., Sun, W. J., Ji, X. M., & Jia, L. F. (2005). Cerebrospinal fluid tau, Abeta1–42 and inflammatory cytokines in patients with Alzheimer’s disease and vascular dementia. Neuroscience Letters, 383, 12–16.
Johnson, N. A., Jahng, G. H., Weiner, M. W., Miller, B. L., Chui, H. C., Jagust, W. J., et al. (2005). Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: Initial experience. Radiology, 234, 851–859.
Kajstura, J., Pertoldi, B., Leri, A., Beltrami, C. A., Deptala, A., Darzynkiewicz, Z., et al. (2000). Telomere shortening is an in vivo marker of myocyte replication and aging. American Journal of Pathology, 156, 813–819.
Kamer, A. R. (2010). Systemic inflammation and disease progression in Alzheimer disease. Neurology, 74, 1157. (author reply 1157–1158).
Kang, M. K., Kameta, A., Baluda, M. A., & Park, N. H. (2003). Telomere shortening does not occur during postmaturational aging in situ in normal human oral fibroblasts. Mechanisms of Ageing and Development, 124, 873–876.
Kaszubowska, L., Kaczor, J. J., Hak, L., Dettlaff-Pokora, A., Szarynska, M., & Kmiec, Z. (2011). Sensitivity of natural killer cells to activation in the process of ageing is related to the oxidative and inflammatory status of the elderly. Journal of Physiology and Pharmacology, 62, 101–109.
Katepalli, M. P., Adams, A. A., Lear, T. L., & Horohov, D. W. (2008). The effect of age and telomere length on immune function in the horse. Developmental and Comparative Immunology, 32, 1409–1415.
Kawanishi, S., & Oikawa, S. (2004). Mechanism of telomere shortening by oxidative stress. Annals of the New York Academy of Sciences, 1019, 278–284.
Kejariwal, D., Stepien, K. M., Smith, T., Kennedy, H., Hughes, D. A., & Sampson, M. J. (2008). Lack of association of colonic epithelium telomere length and oxidative DNA damage in type 2 diabetes under good metabolic control. BioMed Central Endocrine Disorders, 8, 12.
Kiecolt-Glaser, J. K., Gouin, J. P., Weng, N. P., Malarkey, W. B., Beversdorf, D. Q., & Glaser, R. (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine, 73, 16–22.
Kim, J., Lee, S., Bhattacharjee, R., Khalyfa, A., Kheirandish-Gozal, L., & Gozal, D. (2010). Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrations in children with obstructive sleep apnea. Chest, 138, 91–99.
Kim, J. M., Stewart, R., Shin, I. S., & Yoon, J. S. (2002). Low cholesterol, cognitive function and Alzheimer s disease in a community population with cognitive impairment. The Journal of Nutrition, Health & Aging, 6, 320–323.
Kinouchi, Y., Hiwatashi, N., Chida, M., Nagashima, F., Takagi, S., Maekawa, H., et al. (1998). Telomere shortening in the colonic mucosa of patients with ulcerative colitis. Journal of Gastroenterology, 33, 343–348.
Kipling, D., & Cooke, H. J. (1992). Beginning or end? Telomere structure, genetics and biology. Human Molecular Genetics, 1, 3–6.
Kljajevic, V. (2011). From cell to cognition: Can changes in telomere length indicate patterns of cognitive aging? Clinical Science (London), 121, 313–314.
Koistinaho, J., Malm, T., & Goldsteins, G. (2011). Glycogen synthase kinase-3beta: A mediator of inflammation in Alzheimer’s disease? International Journal of Alzheimer’s Disease, 2011, 129753.
Koukolik, F. (1986). The estimated prevalence of severe dementia due to Alzheimer’s disease in the population over 65 years of age in Czechoslovakia in 1983. Casopis Lekaru Ceskych, 125, 1289–1290.
Kruk, P. A., Rampino, N. J., & Bohr, V. A. (1995). DNA damage and repair in telomeres: Relation to aging. Proceedings of the National Academy of Sciences of the United States of America, 92, 258–262.
Kume, K., Hanyu, H., Sato, T., Hirao, K., Kanetaka, H., Shimizu, S., et al. (2010). Prediction of the development of Alzheimer disease in patients with mild cognitive impairment. Nihon Ronen Igakkai Zasshi, 47, 147–152.
Kurz, D. J., Decary, S., Hong, Y., Trivier, E., Akhmedov, A., & Erusalimsky, J. D. (2004). Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. Journal of Cell Science, 117, 2417–2426.
Kveiborg, M., Kassem, M., Langdahl, B., Eriksen, E. F., Clark, B. F., & Rattan, S. I. (1999). Telomere shortening during aging of human osteoblasts in vitro and leukocytes in vivo: Lack of excessive telomere loss in osteoporotic patients. Mechanisms of Ageing and Development, 106, 261–271.
Landegent, J. E., Jansen in de Wal, N., Fisser-Groen, Y. M., Bakker, E., van der Ploeg, M., & Pearson, P. L. (1986). Fine mapping of the Huntington disease linked D4S10 locus by non-radioactive in situ hybridization. Human Genetics, 73, 354–357.
Lecanu, L., Greeson, J., & Papadopoulos, V. (2006). Beta-amyloid and oxidative stress jointly induce neuronal death, amyloid deposits, gliosis, and memory impairment in the rat brain. Pharmacology, 76, 19–33.
Lee, D. Y., Fletcher, E., Martinez, O., Zozulya, N., Kim, J., Tran, J., et al. (2010). Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke, 41, 1791–1797.
Levy, M. Z., Allsopp, R. C., Futcher, A. B., Greider, C. W., & Harley, C. B. (1992). Telomere end-replication problem and cell aging. Journal of Molecular Biology, 225, 951–960.
Li, J., Wang, Y. J., Zhang, M., Xu, Z. Q., Gao, C. Y., Fang, C. Q., et al. (2011). Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology, 76, 1485–1491.
Liu, L., Trimarchi, J. R., Navarro, P., Blasco, M. A., & Keefe, D. L. (2003). Oxidative stress contributes to arsenic-induced telomere attrition, chromosome instability, and apoptosis. Journal of Biological Chemistry, 278, 31998–32004.
Llano, D. A., Laforet, G., & Devanarayan, V. (2011). Derivation of a new ADAS-cog composite using tree-based multivariate analysis: Prediction of conversion from mild cognitive impairment to Alzheimer disease. Alzheimer Disease and Associated Disorders, 25, 73–84.
Lukens, J. N., Van Deerlin, V., Clark, C. M., Xie, S. X., & Johnson, F. B. (2009). Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimer’s & Dementia, 5, 463–469.
Maccioni, R. B., Rojo, L. E., Fernandez, J. A., & Kuljis, R. O. (2009). The role of neuroimmunomodulation in Alzheimer’s disease. Annals of the New York Academy of Sciences, 1153, 240–246.
Maeda, T., Guan, J. Z., Oyama, J., Higuchi, Y., & Makino, N. (2009). Aging-associated alteration of subtelomeric methylation in Parkinson’s disease. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 64, 949–955.
Mainous, A. G., 3rd, Codd, V., Diaz, V. A., Schoepf, U. J., Everett, C. J., Player, M. S., et al. (2010). Leukocyte telomere length and coronary artery calcification. Atherosclerosis, 210, 262–267.
Majores, M., Bagli, M., Papassotiropoulos, A., Schwab, S. G., Jessen, F., Rao, M. L., et al. (2000). Allelic association between the D10S1423 marker and Alzheimer’s disease in a German population. Neuroscience Letters, 289, 224–226.
Makino, N., Maeda, T., Oyama, J., & Higuchi, Y. (2009). Role of the environmental factors on aging. Nihon Rinsho, 67, 1332–1336.
Marchesi, V. T. (2011). Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. Federation of American Societies for Experimental Biology Journal, 25, 5–13.
Marcondes, A. M., Ramakrishnan, A., & Deeg, H. J. (2009). Myeloid malignancies and the marrow microenvironment: Some recent studies in patients with MDS. Current Cancer Therapy Reviews, 5, 310–314.
Martinez, J. A. (2006). Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. Journal of Physiology and Biochemistry, 62, 303–306.
Martinez-Delgado, B., Yanowsky, K., Inglada-Perez, L., Domingo, S., Urioste, M., Osorio, A., et al. (2011). Genetic anticipation is associated with telomere shortening in hereditary breast cancer. PLoS Genetics, 7, e1002182.
Martin-Ruiz, C., Dickinson, H. O., Keys, B., Rowan, E., Kenny, R. A., & Von Zglinicki, T. (2006). Telomere length predicts poststroke mortality, dementia, and cognitive decline. Annals of Neurology, 60, 174–180.
Martin-Ruiz, C., Jagger, C., Kingston, A., Collerton, J., Catt, M., Davies, K., et al. (2011). Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mechanisms of Ageing and Development, 132(10), 496–502.
Masi, S., Salpea, K. D., Li, K., Parkar, M., Nibali, L., Donos, N., et al. (2011). Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radical Biology and Medicine, 50, 730–735.
Mather, K. A., Jorm, A. F., Anstey, K. J., Milburn, P. J., Easteal, S., & Christensen, H. (2010). Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: A population study. BioMed Central Geriatrics, 10, 62.
Matthews, C., Gorenne, I., Scott, S., Figg, N., Kirkpatrick, P., Ritchie, A., et al. (2006). Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circulation Research, 99, 156–164.
Mattson, M. P. (1990). Excitatory amino acids, growth factors, and calcium: A teeter-totter model for neural plasticity and degeneration. Advances in Experimental Medicine and Biology, 268, 211–220.
Mattson, M. P. (1992). Calcium as sculptor and destroyer of neural circuitry. Experimental Gerontology, 27, 29–49.
Maxwell, F., McGlynn, L. M., Muir, H. C., Talwar, D., Benzeval, M., Robertson, T., et al. (2011). Telomere attrition and decreased fetuin-a levels indicate accelerated biological aging and are implicated in the pathogenesis of colorectal cancer. Clinical Cancer Research, 17, 5573–5581.
McPhie, D. L., Coopersmith, R., Hines-Peralta, A., Chen, Y., Ivins, K. J., Manly, S. P., et al. (2003). DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. Journal of Neuroscience, 23, 6914–6927.
Meeker, A. K., Hicks, J. L., Platz, E. A., March, G. E., Bennett, C. J., Delannoy, M. J., et al. (2002). Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Research, 62, 6405–6409.
Melk, A., Kittikowit, W., Sandhu, I., Halloran, K. M., Grimm, P., Schmidt, B. M., et al. (2003). Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney International, 63, 2134–2143.
Melk, A., Ramassar, V., Helms, L. M., Moore, R., Rayner, D., Solez, K., et al. (2000). Telomere shortening in kidneys with age. Journal of the American Society of Nephrology, 11, 444–453.
Meresse, B., Dubucquoi, S., Tourvieille, B., Desreumaux, P., Colombel, J. F., & Dessaint, J. P. (2001). CD28+ intraepithelial lymphocytes with long telomeres are recruited within the inflamed ileal mucosa in Crohn disease. Human Immunology, 62, 694–700.
Merino, A., Buendia, P., Martin-Malo, A., Aljama, P., Ramirez, R., & Carracedo, J. (2011). Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J Immunol., 186, 1809–1815.
Metcalfe, M. J., & Figueiredo-Pereira, M. E. (2010). Relationship between tau pathology and neuroinflammation in Alzheimer’s disease. Mount Sinai Journal of Medicine, 77, 50–58.
Mhatre, M., Floyd, R. A., & Hensley, K. (2004). Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. Journal of Alzheimer’s Disease, 6, 147–157.
Milwain, E. J., & Nagy, Z. (2005). Depressive symptoms increase the likelihood of cognitive impairment in elderly people with subclinical Alzheimer pathology. Dementia and Geriatric Cognitive Disorders, 19, 46–50.
Mirabello, L., Huang, W. Y., Wong, J. Y., Chatterjee, N., Reding, D., Crawford, E. D., et al. (2009). The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell, 8, 405–413.
Mirabello, L., Richards, E. G., Duong, L. M., Yu, K., Wang, Z., Cawthon, R., et al. (2011). Telomere length and variation in telomere biology genes in individuals with osteosarcoma. International Journal of Molecular Epidemiology and Genetics, 2, 19–29.
Mirabello, L., Yu, K., Kraft, P., De Vivo, I., Hunter, D. J., Prescott, J., et al. (2010). The association of telomere length and genetic variation in telomere biology genes. Human Mutation, 31, 1050–1058.
Misonou, H., Morishima-Kawashima, M., & Ihara, Y. (2000). Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry, 39, 6951–6959.
Modrego, P. J., & Ferrandez, J. (2004). Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: A prospective cohort study. Archives of Neurology, 61, 1290–1293.
Mu, Y., Zhang, Q., Mei, L., Liu, X., Yang, W., & Yu, J. (2012). Telomere shortening occurs early during gastrocarcinogenesis. Medical Oncology, 29(2), 893–898.
Murillo-Ortiz, B., Albarran-Tamayo, F., Arenas-Aranda, D., Benitez-Bribiesca, L., Malacara-Hernandez, J., Martinez-Garza, S., et al. (2012). Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male, 15(1), 54–58.
Murnane, J. P. (2006). Telomeres and chromosome instability. DNA Repair (Amsterdam), 5, 1082–1092.
Naert, G., & Rivest, S. (2011). CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Journal of Neuroscience, 31, 6208–6220.
Nakajima, T., Nakashima, T., Okada, Y., Jo, M., Nishikawa, T., Mitsumoto, Y., et al. (2010). Nuclear size measurement is a simple method for the assessment of hepatocellular aging in non-alcoholic fatty liver disease: Comparison with telomere-specific quantitative FISH and p21 immunohistochemistry. Pathology International, 60, 175–183.
Nawrot, T. S., & Staessen, J. A. (2008). Genetic variation and environmental factors in biological and arterial ageing. Verhandelingen - Koninklijke Academie voor Geneeskunde van Belgie, 70, 323–338.
Nettleton, J. A., Diez-Roux, A., Jenny, N. S., Fitzpatrick, A. L., & Jacobs, D. R., Jr. (2008). Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). American Journal of Clinical Nutrition, 88, 1405–1412.
Niemann, B., Chen, Y., Teschner, M., Li, L., Silber, R. E., & Rohrbach, S. (2011). Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. Journal of the American College of Cardiology, 57, 577–585.
Nothias, F., Cadusseau, J., Dusart, I., & Peschanski, M. (1991). Fetal neural transplants into an area of neurodegeneration in the spinal cord of the adult rat. Restorative Neurology and Neuroscience, 2, 283–288.
Nowak, R., Siwicki, J. K., Chechlinska, M., & Markowicz, S. (2002). Telomere shortening and atherosclerosis. Lancet, 359, 976. (author reply 976–977).
Obana, N., Takagi, S., Kinouchi, Y., Tokita, Y., Sekikawa, A., Takahashi, S., et al. (2003). Telomere shortening of peripheral blood mononuclear cells in coronary disease patients with metabolic disorders. Internal Medicine, 42, 150–153.
Obermayr, R. P., Mayerhofer, L., Knechtelsdorfer, M., Tragl, K. H., & Geyer, G. (2003). The reduced release of GH by GHRH in 8 subjects aged 65–69 years is augmented considerably by rivastigmine, a drug for Alzheimer’s disease. Gerontology, 49, 191–195.
Oikawa, S., & Kawanishi, S. (1999). Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. Federation of European Biochemical Societies Letters, 453, 365–368.
Olivieri, F., Lorenzi, M., Antonicelli, R., Testa, R., Sirolla, C., Cardelli, M., et al. (2009). Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis, 206, 588–593.
O’Sullivan, J. N., Bronner, M. P., Brentnall, T. A., Finley, J. C., Shen, W. T., Emerson, S., et al. (2002). Chromosomal instability in ulcerative colitis is related to telomere shortening. Nature Genetics, 32, 280–284.
Ozsarlak-Sozer, G., Kerry, Z., Gokce, G., Oran, I., & Topcu, Z. (2011). Oxidative stress in relation to telomere length maintenance in vascular smooth muscle cells following balloon angioplasty. Journal of Physiology and Biochemistry, 67, 35–42.
Pallas, M., Camins, A., Smith, M. A., Perry, G., Lee, H. G., & Casadesus, G. (2008). From aging to Alzheimer’s disease: Unveiling “the switch” with the senescence-accelerated mouse model (SAMP8). Journal of Alzheimer’s Disease, 15, 615–624.
Palmer, K., Backman, L., Winblad, B., & Fratiglioni, L. (2008). Mild cognitive impairment in the general population: Occurrence and progression to Alzheimer disease. The American Journal of Geriatric Psychiatry, 16, 603–611.
Palmer, K., Berger, A. K., Monastero, R., Winblad, B., Backman, L., & Fratiglioni, L. (2007). Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology, 68, 1596–1602.
Panossian, L. A., Porter, V. R., Valenzuela, H. F., Zhu, X., Reback, E., Masterman, D., et al. (2003). Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiology of Aging, 24, 77–84.
Panza, F., Capurso, C., D’Introno, A., Colacicco, A. M., Capurso, A., Solfrizzi, V., et al. (2007). Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology, 68, 964. (author replly 964–965).
Paul, L. (2011). Diet, nutrition and telomere length. The Journal of Nutritional Biochemistry, 22(10), 895–901.
Pedersen-Lane, J. H., Zurier, R. B., & Lawrence, D. A. (2007). Analysis of the thiol status of peripheral blood leukocytes in rheumatoid arthritis patients. Journal of Leukocyte Biology, 81, 934–941.
Pelliccia, F., Bosco, N., & Rocchi, A. (2010). Breakages at common fragile sites set boundaries of amplified regions in two leukemia cell lines K562—Molecular characterization of FRA2H and localization of a new CFS FRA2S. Cancer Letters, 299, 37–44.
Peng, G., & Lin, S. Y. (2009). The linkage of chromatin remodeling to genome maintenance: Contribution from a human disease gene BRIT1/MCPH1. Epigenetics, 4, 457–461.
Perona, R. (2010). The Nobel Prize in physiology or medicine 2009 “for telomere biology” and its relevance to cancer and related diseases. Clinical and Translational Oncology, 12, 647–649.
Peters, O., Schipke, C. G., Philipps, A., Haas, B., Pannasch, U., Wang, L. P., et al. (2009). Astrocyte function is modified by Alzheimer’s disease-like pathology in aged mice. Journal of Alzheimer’s Disease, 18, 177–189.
Pimplikar, S. W. (2009). Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. International Journal of Biochemistry & Cell Biology, 41, 1261–1268.
Polychronopoulou, S., & Koutroumba, P. (2004). Telomere length variation and telomerase activity expression in patients with congenital and acquired aplastic anemia. Acta Haematologica, 111, 125–131.
Pritchard, C., Casher, D., Bull, L., Cox, D. R., & Myers, R. M. (1990). A cloned DNA segment from the telomeric region of human chromosome 4p is not detectably rearranged in Huntington disease patients. Proceedings of the National Academy of Sciences of the United States of America, 87, 7309–7313.
Proctor, C. J., & Kirkwood, T. B. (2002). Modelling telomere shortening and the role of oxidative stress. Mechanisms of Ageing and Development, 123, 351–363.
Ptok, U., Papassotiropoulos, A., Maier, W., & Heun, R. (2000). Advanced parental age: a risk factor for Alzheimer’s disease or depression in the elderly? International Psychogeriatrics, 12, 445–451.
Qi, L., Strong, M. A., Karim, B. O., Huso, D. L., & Greider, C. W. (2005). Telomere fusion to chromosome breaks reduces oncogenic translocations and tumour formation. Nature Cell Biology, 7, 706–711.
Quadri, P., Fragiacomo, C., Pezzati, R., Zanda, E., Forloni, G., Tettamanti, M., et al. (2004). Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. American Journal of Clinical Nutrition, 80, 114–122.
Ragno M, Pianese L, Pinelli M, Silvestri S, Cacchio G, Di Marzio F, Scarcella M, Coretti F, Altamura F, Monticelli A, Castaldo I (2011) Shorter telomeres in patients with cerebral autosomal dominant arteriopathy and leukoencephalopathy (CADASIL). Neurogenetics.
Rajaraman, S., Choi, J., Cheung, P., Beaudry, V., Moore, H., & Artandi, S. E. (2007). Telomere uncapping in progenitor cells with critical telomere shortening is coupled to S-phase progression in vivo. Proceedings of the National Academy of Sciences of the United States of America, 104, 17747–17752.
Rampazzo, E., Bertorelle, R., Serra, L., Terrin, L., Candiotto, C., Pucciarelli, S., et al. (2010). Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. British Journal of Cancer, 102, 1300–1305.
Ready, R. E., Ott, B. R., Grace, J., & Cahn-Weiner, D. A. (2003). Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. The American Journal of Geriatric Psychiatry, 11, 222–228.
Reale, M., Iarlori, C., Feliciani, C., & Gambi, D. (2008). Peripheral chemokine receptors, their ligands, cytokines and Alzheimer’s disease. Journal of Alzheimer’s Disease, 14, 147–159.
Reeve, A., Norman, A., Sinclair, P., Whittington-Smith, R., Hamey, Y., Donnai, D., et al. (1993). True telomeric translocation in a baby with the Prader-Willi phenotype. American Journal of Medical Genetics, 47, 1–6.
Ren, F., Li, C., Xi, H., Wen, Y., & Huang, K. (2009). Estimation of human age according to telomere shortening in peripheral blood leukocytes of Tibetan. American Journal of Forensic Medicine and Pathology, 30, 252–255.
Resende, R., Moreira, P. I., Proenca, T., Deshpande, A., Busciglio, J., Pereira, C., et al. (2008). Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radical Biology and Medicine, 44, 2051–2057.
Ricchieri, G. L., Argentiero, V., Ongaro, G., & Tavolato, B. (1983). The complement system and plasma protein levels in Alzheimer’s disease. Acta neurologica (Napoli), 5, 103–108.
Richards, J. B., Valdes, A. M., Gardner, J. P., Kato, B. S., Siva, A., Kimura, M., et al. (2008). Homocysteine levels and leukocyte telomere length. Atherosclerosis, 200, 271–277.
Richter, T., & von Zglinicki, T. (2007). A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Experimental Gerontology, 42, 1039–1042.
Riou, J. F., Guittat, L., Mailliet, P., Laoui, A., Renou, E., Petitgenet, O., et al. (2002). Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proceedings of the National Academy of Sciences of the United States of America, 99, 2672–2677.
Risques, R. A., Lai, L. A., Brentnall, T. A., Li, L., Feng, Z., Gallaher, J., et al. (2008). Ulcerative colitis is a disease of accelerated colon aging: Evidence from telomere attrition and DNA damage. Gastroenterology, 135, 410–418.
Risques, R. A., Lai, L. A., Himmetoglu, C., Ebaee, A., Li, L., Feng, Z., et al. (2011). Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Research, 71, 1669–1679.
Risques, R. A., Vaughan, T. L., Li, X., Odze, R. D., Blount, P. L., Ayub, K., et al. (2007). Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiology, Biomarkers & Prevention, 16, 2649–2655.
Robbins, C., Theilmann, J., Youngman, S., Haines, J., Altherr, M. J., Harper, P. S., et al. (1989). Evidence from family studies that the gene causing Huntington disease is telomeric to D4S95 and D4S90. American Journal of Human Genetics, 44, 422–425.
Rolyan, H., Scheffold, A., Heinrich, A., Begus-Nahrmann, Y., Langkopf, B. H., Holter, S. M., et al. (2011). Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain, 134, 2044–2056.
Rosi, S., Ramirez-Amaya, V., Hauss-Wegrzyniak, B., & Wenk, G. L. (2004). Chronic brain inflammation leads to a decline in hippocampal NMDA-R1 receptors. J Neuroinflammation, 1, 12.
Salpea, K. D., Talmud, P. J., Cooper, J. A., Maubaret, C. G., Stephens, J. W., Abelak, K., et al. (2010). Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis, 209, 42–50.
Samani, N. J., Boultby, R., Butler, R., Thompson, J. R., & Goodall, A. H. (2001). Telomere shortening in atherosclerosis. Lancet, 358, 472–473.
Sampson, M. J., Winterbone, M. S., Hughes, J. C., Dozio, N., & Hughes, D. A. (2006). Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care, 29, 283–289.
Sanders, J. L., Cauley, J. A., Boudreau, R. M., Zmuda, J. M., Strotmeyer, E. S., Opresko, P. L., et al. (2009). Leukocyte telomere length is not associated with BMD, osteoporosis, or fracture in older adults: Results from the health, aging and body composition study. Journal of Bone and Mineral Research, 24, 1531–1536.
Sando, S. B., Melquist, S., Cannon, A., Hutton, M. L., Sletvold, O., Saltvedt, I., et al. (2008). APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central Norway. BioMed Central Neurology, 8, 9.
Saretzki, G., Murphy, M. P., & von Zglinicki, T. (2003). MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell, 2, 141–143.
Saretzki, G., & von Zglinicki, T. (1999). Replicative senescence as a model of aging: The role of oxidative stress and telomere shortening—An overview. Zeitschrift fur Gerontologie und Geriatrie, 32, 69–75.
Sastry, P. S., & Parikh, P. (2003). The earlier age of onset of malignancy in developing world is related to overall infection burden and could be due to the effect on telomere length. Medical Hypotheses, 60, 573–574.
Satoh, M., Ishikawa, Y., Takahashi, Y., Itoh, T., Minami, Y., & Nakamura, M. (2008). Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis, 198, 347–353.
Satoh, M., Minami, Y., Takahashi, Y., Tabuchi, T., Itoh, T., & Nakamura, M. (2009). Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clinical Science (London), 116, 827–835.
Savale, L., Chaouat, A., Bastuji-Garin, S., Marcos, E., Boyer, L., Maitre, B., et al. (2009). Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 179, 566–571.
Sawyer, J. R., Goosen, L. S., Stine, K. C., & Thomas, J. R. (1994). Telomere fusion as a mechanism for the progressive loss of the short arm of chromosome 11 in an anaplastic Wilms’ tumor. Cancer, 74, 767–773.
Sebastian, C., Herrero, C., Serra, M., Lloberas, J., Blasco, M. A., & Celada, A. (2009). Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. Journal of Immunology, 183, 2356–2364.
Sekoguchi, S., Nakajima, T., Moriguchi, M., Jo, M., Nishikawa, T., Katagishi, T., et al. (2007). Role of cell-cycle turnover and oxidative stress in telomere shortening and cellular senescence in patients with chronic hepatitis C. Journal of Gastroenterology and Hepatology, 22, 182–190.
Serra, V., Grune, T., Sitte, N., Saretzki, G., & von Zglinicki, T. (2000). Telomere length as a marker of oxidative stress in primary human fibroblast cultures. Annals of the New York Academy of Sciences, 908, 327–330.
Shen, J., Gammon, M. D., Terry, M. B., Wang, Q., Bradshaw, P., Teitelbaum, S. L., et al. (2009). Telomere length, oxidative damage, antioxidants and breast cancer risk. International Journal of Cancer, 124, 1637–1643.
Shiels, P. G., McGlynn, L. M., Macintyre, A., Johnson, P. C., Batty, G. D., Burns, H., et al. (2011). Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS ONE, 6, e22521.
Shlush, L. I., Skorecki, K. L., Itzkovitz, S., Yehezkel, S., Segev, Y., Shachar, H., et al. (2011). Telomere elongation followed by telomere length reduction, in leukocytes from divers exposed to intense oxidative stress—Implications for tissue and organismal aging. Mechanisms of Ageing and Development, 132, 123–130.
Silva, P. N., Gigek, C. O., Leal, M. F., Bertolucci, P. H., de Labio, R. W., Payao, S. L., et al. (2008). Promoter methylation analysis of SIRT3, SMARCA5, HTERT and CDH1 genes in aging and Alzheimer’s disease. Journal of Alzheimer’s Disease, 13, 173–176.
Simpson, J. E., Ince, P. G., Haynes, L. J., Theaker, R., Gelsthorpe, C., Baxter, L., et al. (2010). Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer-type pathology in the ageing brain. Neuropathology and Applied Neurobiology, 36, 25–40.
Singh, M., Nam, D. T., Arseneault, M., & Ramassamy, C. (2010). Role of by-products of lipid oxidation in Alzheimer’s disease brain: A focus on acrolein. Journal of Alzheimer’s Disease, 21, 741–756.
Sisodia, S. S., & Price, D. L. (1995). Role of the beta-amyloid protein in Alzheimer’s disease. Federation of American Societies for Experimental Biology Journal, 9, 366–370.
Sloand, E. M., Pfannes, L., Ling, C., Feng, X., Jasek, M., Calado, R., et al. (2010). Graft-versus-host disease: Role of inflammation in the development of chromosomal abnormalities of keratinocytes. Biology of Blood and Marrow Transplantation, 16, 1665–1673.
Smith, M. A., Hirai, K., Hsiao, K., Pappolla, M. A., Harris, P. L., Siedlak, S. L., et al. (1998). Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. Journal of Neurochemistry, 70, 2212–2215.
Smith, M. A., Zhu, X., Tabaton, M., Liu, G., McKeel, D. W., Jr., Cohen, M. L., et al. (2010). Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. Journal of Alzheimer’s Disease, 19, 363–372.
Sonobe, N., Hata, R., Ishikawa, T., Sonobe, K., Matsumoto, T., Toyota, Y., et al. (2010). Risk of progression from mild memory impairment to clinically diagnosable Alzheimer’s disease in a Japanese community (from the Nakayama Study). International Psychogeriatrics, 1–8.
Sozou, P. D., & Kirkwood, T. B. (2001). A stochastic model of cell replicative senescence based on telomere shortening, oxidative stress, and somatic mutations in nuclear and mitochondrial DNA. Journal of Theoretical Biology, 213, 573–586.
Spyridopoulos, I., Hoffmann, J., Aicher, A., Brummendorf, T. H., Doerr, H. W., Zeiher, A. M., et al. (2009). Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: Role of cytomegalovirus seropositivity. Circulation, 120, 1364–1372.
Srikanth, V., Maczurek, A., Phan, T., Steele, M., Westcott, B., Juskiw, D., et al. (2011). Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiology of Aging, 32, 763–777.
Standridge, J. B. (2006). Vicious cycles within the neuropathophysiologic mechanisms of Alzheimer’s disease. Current Alzheimer Research, 3, 95–108.
Starr, J. M., Shiels, P. G., Harris, S. E., Pattie, A., Pearce, M. S., Relton, C. L., et al. (2008). Oxidative stress, telomere length and biomarkers of physical aging in a cohort aged 79 years from the 1932 Scottish Mental Survey. Mechanisms of Ageing and Development, 129, 745–751.
Steer, S. E., Williams, F. M., Kato, B., Gardner, J. P., Norman, P. J., Hall, M. A., et al. (2007). Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Annals of the Rheumatic Diseases, 66, 476–480.
Takagi, S., Kinouchi, Y., Hiwatashi, N., Hirai, M., Suzuki, S., Takahashi, S., et al. (2002). Correlative polymorphism of NAD(P)H: Quinone oxidoreductase (NQO1) with telomere shortening in colorectal cancer. Anticancer Research, 22, 2749–2752.
Takagi, S., Kinouchi, Y., Hiwatashi, N., Nagashima, F., Chida, M., Takahashi, S., et al. (2000). Relationship between microsatellite instability and telomere shortening in colorectal cancer. Diseases of the Colon and Rectum, 43, S12–S17.
Takasaki, T., Tsuji, A., Ikeda, N., & Ohishi, M. (2003). Age estimation in dental pulp DNA based on human telomere shortening. International Journal of Legal Medicine, 117, 232–234.
Talelli, P., & Ellul, J. (2004). Are patients with cognitive impairment after stroke at increased risk for developing Alzheimer disease? Archives of Neurology, 61, 983. (author reply 983).
Tamagno, E., Guglielmotto, M., Aragno, M., Borghi, R., Autelli, R., Giliberto, L., et al. (2008). Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. Journal of Neurochemistry, 104, 683–695.
Tanaka, Y., Moritoh, Y., & Miwa, N. (2007). Age-dependent telomere-shortening is repressed by phosphorylated alpha-tocopherol together with cellular longevity and intracellular oxidative-stress reduction in human brain microvascular endotheliocytes. Journal of Cellular Biochemistry, 102, 689–703.
Tanzi, R. E., Haines, J. L., Watkins, P. C., Stewart, G. D., Wallace, M. R., Hallewell, R., et al. (1988). Genetic linkage map of human chromosome 21. Genomics, 3, 129–136.
Tanzi, R. E., Watkins, P. C., Stewart, G. D., Wexler, N. S., Gusella, J. F., & Haines, J. L. (1992). A genetic linkage map of human chromosome 21: Analysis of recombination as a function of sex and age. American Journal of Human Genetics, 50, 551–558.
Tarry-Adkins, J. L., Martin-Gronert, M. S., Chen, J. H., Cripps, R. L., & Ozanne, S. E. (2008). Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. Federation of American Societies for Experimental Biology Journal, 22, 2037–2044.
Tchirkov, A., & Lansdorp, P. M. (2003). Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Human Molecular Genetics, 12, 227–232.
Teyssier, J. R., Ragot, S., Donzel, A., & Chauvet-Gelinier, J. C. (2010). Telomeres in the brain cortex of depressive patients. Encephale, 36, 491–494.
Theilmann, J., Kanani, S., Shiang, R., Robbins, C., Quarrell, O., Huggins, M., et al. (1989). Non-random association between alleles detected at D4S95 and D4S98 and the Huntington’s disease gene. Journal of Medical Genetics, 26, 676–681.
Thomas, P., & Fenech, M. (2007). A review of genome mutation and Alzheimer’s disease. Mutagenesis, 22, 15–33.
Thomas, P., NJ, O. C., & Fenech, M. (2008). Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mechanisms of Ageing and Development, 129, 183–190.
Thorsteinsdottir, S., Gudjonsson, T., Nielsen, O. H., Vainer, B., & Seidelin, J. B. (2011). Pathogenesis and biomarkers of carcinogenesis in ulcerative colitis. Nature Reviews. Gastroenterology & Hepatology, 8, 395–404.
Tomac, A. C., & Hoffer, B. J. (2001). Assignment of the mouse Park2 (PARKIN), the homologue to a new human Parkinson candidate gene, to the telomeric region of mouse 17A3.2–3.3, by in situ hybridization. Cytogenetics and Cell Genetics, 95, 120–121.
Town, T. (2010). Inflammation, immunity, and Alzheimer’s disease. CNS & Neurological Disorders: Drug Targets, 9, 129–131.
Tsolou, A., Passos, J. F., Nelson, G., Arai, Y., & Zglinicki, T. (2008). ssDNA fragments induce cell senescence by telomere uncapping. Experimental Gerontology, 43, 892–899.
Tsuji, A., Ishiko, A., Takasaki, T., & Ikeda, N. (2002). Estimating age of humans based on telomere shortening. Forensic Science International, 126, 197–199.
Tuppo, E. E., & Forman, L. J. (2001). Free radical oxidative damage and Alzheimer’s disease. Journal of the American Osteopathic Association, 101, S11–S15.
Uziel, O., Beery, E., Dronichev, V., Samocha, K., Gryaznov, S., Weiss, L., et al. (2010). Telomere shortening sensitizes cancer cells to selected cytotoxic agents: In vitro and in vivo studies and putative mechanisms. PLoS ONE, 5, e9132.
Uziel, O., Reshef, H., Ravid, A., Fabian, I., Halperin, D., Ram, R., et al. (2008). Oxidative stress causes telomere damage in Fanconi anaemia cells—A possible predisposition for malignant transformation. British Journal of Haematology, 142, 82–93.
Valdes, A. M., Andrew, T., Gardner, J. P., Kimura, M., Oelsner, E., Cherkas, L. F., et al. (2005). Obesity, cigarette smoking, and telomere length in women. Lancet, 366, 662–664.
Valdes, A. M., Deary, I. J., Gardner, J., Kimura, M., Lu, X., Spector, T. D., et al. (2010). Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiology of Aging, 31, 986–992.
van der Harst, P., de Boer, R. A., Samani, N. J., Wong, L. S., Huzen, J., Codd, V., et al. (2010). Telomere length and outcome in heart failure. Annals of Medicine, 42, 36–44.
van der Harst, P., de Boer, R. A., & van Veldhuisen, D. J. (2009). The Nobel Prize for medicine for telomere biology and relevance to heart failure research. European Journal of Heart Failure, 11, 1113–1115.
van der Harst, P., Wong, L. S., de Boer, R. A., Brouilette, S. W., van der Steege, G., Voors, A. A., et al. (2008). Possible association between telomere length and renal dysfunction in patients with chronic heart failure. American Journal of Cardiology, 102, 207–210.
van Exel, E., Eikelenboom, P., Comijs, H., Frolich, M., Smit, J. H., Stek, M. L., et al. (2009). Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Archives of General Psychiatry, 66, 1263–1270.
van Rossum, I. A., Vos, S., Handels, R., & Visser, P. J. (2010). Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: Implications for trial design. Journal of Alzheimer’s Disease, 20, 881–891.
Vasan, R. S., Demissie, S., Kimura, M., Cupples, L. A., Rifai, N., White, C., et al. (2008). Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: The Framingham Heart Study. Circulation, 117, 1138–1144.
Viel, T. A., & Buck, H. S. (2011). Kallikrein-kinin system mediated inflammation in Alzheimer’s disease in vivo. Current Alzheimer Research, 8, 59–66.
Vijayan, V. K., Geddes, J. W., Anderson, K. J., Chang-Chui, H., Ellis, W. G., & Cotman, C. W. (1991). Astrocyte hypertrophy in the Alzheimer’s disease hippocampal formation. Experimental Neurology, 112, 72–78.
Viscardi, V., Clerici, M., Cartagena-Lirola, H., & Longhese, M. P. (2005). Telomeres and DNA damage checkpoints. Biochimie, 87, 613–624.
Vodenicharov, M. D., & Wellinger, R. J. (2007). The cell division cycle puts up with unprotected telomeres: Cell cycle regulated telomere uncapping as a means to achieve telomere homeostasis. Cell Cycle, 6, 1161–1167.
Voghel, G., Thorin-Trescases, N., Farhat, N., Nguyen, A., Villeneuve, L., Mamarbachi, A. M., et al. (2007). Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mechanisms of Ageing and Development, 128, 662–671.
von Bernhardi, R. (2007). Glial cell dysregulation: A new perspective on Alzheimer disease. Neurotoxicity Research, 12, 215–232.
von Zglinicki, T. (2000). Role of oxidative stress in telomere length regulation and replicative senescence. Annals of the New York Academy of Sciences, 908, 99–110.
von Zglinicki, T., Serra, V., Lorenz, M., Saretzki, G., Lenzen-Grossimlighaus, R., Gessner, R., et al. (2000). Short telomeres in patients with vascular dementia: An indicator of low antioxidative capacity and a possible risk factor? Laboratory Investigation, 80, 1739–1747.
Vukovic, B., Beheshti, B., Park, P., Lim, G., Bayani, J., Zielenska, M., et al. (2007). Correlating breakage-fusion-bridge events with the overall chromosomal instability and in vitro karyotype evolution in prostate cancer. Cytogenetic and Genome Research, 116, 1–11.
Vulliamy, T., Marrone, A., Szydlo, R., Walne, A., Mason, P. J., & Dokal, I. (2004). Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nature Genetics, 36, 447–449.
Wan, L., Nie, G., Zhang, J., Luo, Y., Zhang, P., Zhang, Z., et al. (2011). beta-Amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radical Biology and Medicine, 50, 122–129.
Wang, Y. Y., Chen, A. F., Wang, H. Z., Xie, L. Y., Sui, K. X., & Zhang, Q. Y. (2011a). Association of shorter mean telomere length with large artery stiffness in patients with coronary heart disease. Aging Male, 14, 27–32.
Wang, C., Li, J., Liu, Q., Yang, R., Zhang, J. H., Cao, Y. P., et al. (2011b). Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-kappaB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neuroscience Letters, 491, 127–132.
Watfa, G., Dragonas, C., Brosche, T., Dittrich, R., Sieber, C. C., Alecu, C., et al. (2011). Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. The Journal of Nutrition, Health & Aging, 15, 277–281.
Widmann, T. A., Herrmann, M., Taha, N., Konig, J., & Pfreundschuh, M. (2007). Short telomeres in aggressive non-Hodgkin’s lymphoma as a risk factor in lymphomagenesis. Experimental Hematology, 35, 939–946.
Wikgren, M., Karlsson, T., Nilbrink, T., Nordfjall, K., Hultdin, J., Sleegers, K., et al. (2012). APOE epsilon4 is associated with longer telomeres, and longer telomeres among epsilon4 carriers predicts worse episodic memory. Neurobiology of Aging, 33(2), 335–344.
Willeit, P., Willeit, J., Brandstatter, A., Ehrlenbach, S., Mayr, A., Gasperi, A., et al. (2010). Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 1649–1656.
Wolkowitz, O. M., Mellon, S. H., Epel, E. S., Lin, J., Dhabhar, F. S., Su, Y., et al. (2011). Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress-preliminary findings. PLoS ONE, 6, e17837.
Wolkowitz, O. M., Mellon, S. H., Epel, E. S., Lin, J., Reus, V. I., Rosser, R., et al. (2012). Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Molecular Psychiatry, 17(2), 164–172.
Wong, L. S., Huzen, J., van der Harst, P., de Boer, R. A., Codd, V., Westenbrink, B. D., et al. (2010). Anaemia is associated with shorter leucocyte telomere length in patients with chronic heart failure. European Journal of Heart Failure, 12, 348–353.
Yaffe, K., Lindquist, K., Kluse, M., Cawthon, R., Harris, T., Hsueh, W. C., et al. (2011). Telomere length and cognitive function in community-dwelling elders: Findings from the Health ABC Study. Neurobiology of Aging, 32(11), 2055–2060.
Yamada, N. (2003). Telomere shortening, atherosclerosis, and metabolic syndrome. Internal Medicine, 42, 135–136.
Yan, F. L., Zheng, Y., & Zhao, F. D. (2008). Effects of ginkgo biloba extract EGb761 on expression of RAGE and LRP-1 in cerebral microvascular endothelial cells under chronic hypoxia and hypoglycemia. Acta Neuropathologica, 116, 529–535.
Yao, Y., Chinnici, C., Tang, H., Trojanowski, J. Q., Lee, V. M., & Pratico, D. (2004). Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis. Journal of Neuroinflammation, 1, 21.
Yatin, S. M., Varadarajan, S., Link, C. D., & Butterfield, D. A. (1999). In vitro and in vivo oxidative stress associated with Alzheimer’s amyloid beta-peptide (1–42). Neurobiology of Aging, 20, 325–330. (discussion 339–342).
Young, N. S. (2010). Telomere biology and telomere diseases: Implications for practice and research. Hematology American Society of Hematology Education Program Book, 2010, 30–35.
Yudoh, K., Nguyen, T., Nakamura, H., Hongo-Masuko, K., Kato, T., & Nishioka, K. (2005). Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Research & Therapy, 7, R380–R391.
Zee, R. Y., Castonguay, A. J., Barton, N. S., Germer, S., & Martin, M. (2010a). Mean leukocyte telomere length shortening and type 2 diabetes mellitus: A case-control study. Translational Research, 155, 166–169.
Zee, R. Y., Castonguay, A. J., Barton, N. S., & Ridker, P. M. (2010b). Relative leukocyte telomere length and risk of incident ischemic stroke in men: A prospective, nested case-control approach. Rejuvenation Research, 13, 411–414.
Zee, R. Y., Ridker, P. M., & Chasman, D. I. (2011). Genetic variants in eleven telomere-associated genes and the risk of incident cardio/cerebrovascular disease: The Women’s Genome Health Study. Clinica Chimica Acta, 412, 199–202.
Zekry, D., Herrmann, F. R., Irminger-Finger, I., Graf, C., Genet, C., Vitale, A. M., et al. (2010a). Telomere length and ApoE polymorphism in mild cognitive impairment, degenerative and vascular dementia. Journal of the Neurological Sciences, 299, 108–111.
Zekry, D., Herrmann, F. R., Irminger-Finger, I., Ortolan, L., Genet, C., Vitale, A. M., et al. (2010b). Telomere length is not predictive of dementia or MCI conversion in the oldest old. Neurobiology of Aging, 31, 719–720.
Zhai, G., Aviv, A., Hunter, D. J., Hart, D. J., Gardner, J. P., Kimura, M., et al. (2006). Reduction of leucocyte telomere length in radiographic hand osteoarthritis: A population-based study. Annals of the Rheumatic Diseases, 65, 1444–1448.
Zhang, B., Chen, L., Swartz, K. R., Bruemmer, D., Eum, S. Y., Huang, W., et al. (2010). Deficiency of telomerase activity aggravates the blood-brain barrier disruption and neuroinflammatory responses in a model of experimental stroke. Journal of Neuroscience Research, 88, 2859–2868.
Zhang, J., & Ju, Z. (2010). Telomere, DNA damage, and oxidative stress in stem cell aging. Birth Defects Research Part C Embryo Today, 90, 297–307.
Zhang, J., Kong, Q., Zhang, Z., Ge, P., Ba, D., & He, W. (2003). Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cognitive and Behavioral Neurology, 16, 170–176.
Zhang, B., Matsunaga, A., Saku, K., Nakano, S., & Yamada, T. (2004). Associations among plasma lipoprotein subfractions as characterized by analytical capillary isotachophoresis, apolipoprotein E phenotype, Alzheimer disease, and mild cognitive impairment. Arteriosclerosis, Thrombosis, and Vascular Biology, 24, e144–e146.
Zhiyou, C., Yong, Y., Shanquan, S., Jun, Z., Liangguo, H., Ling, Y., et al. (2009). Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer’s disease. Neurochemical Research, 34, 1226–1235.
Zhou, J. M., Zhu, X. F., Lu, Y. J., Deng, R., Huang, Z. S., Mei, Y. P., et al. (2006). Senescence and telomere shortening induced by novel potent G-quadruplex interactive agents, quindoline derivatives, in human cancer cell lines. Oncogene, 25, 503–511.
Zhu, X., Rottkamp, C. A., Boux, H., Takeda, A., Perry, G., & Smith, M. A. (2000). Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 59, 880–888.
Zhu, X., Smith, M. A., Honda, K., Aliev, G., Moreira, P. I., Nunomura, A., et al. (2007). Vascular oxidative stress in Alzheimer disease. Journal of the Neurological Sciences, 257, 240–246.
Zvereva, M. I., Shcherbakova, D. M., & Dontsova, O. A. (2010). Telomerase: Structure, functions, and activity regulation. Biochemistry (Moscow), 75, 1563–1583.
Acknowledgments
This work was supported by the National Nature Science Foundation of China Grant (81070878/H0902) to Prof. Bin Zhao.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, Z., Yan, LJ. & Ratka, A. Telomere Shortening and Alzheimer’s Disease. Neuromol Med 15, 25–48 (2013). https://doi.org/10.1007/s12017-012-8207-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-012-8207-9