Abstract
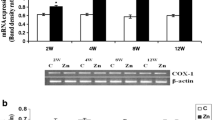
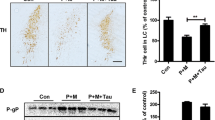
Dopaminergic cells in the substantia nigra are highly vulnerable to the neurodegenerative process of Parkinson’s disease. Therefore, mechanisms that enhance their susceptibility to injury bear important implications for disease pathogenesis. We have previously shown that chronic dichlorvos exposure caused nigrostriatal dopaminergic degeneration and significant behavioral impairments. In this study, we analyzed the relationship between microglial activation and dopaminergic neurodegeneration to examine the possibility that neuroinflammation may induce dopaminergic neuronal loss in the nigrostriatal system. Chronic dichlorvos exposure causes microglial activation including induction of NADPH oxidase and a selective loss of dopaminergic neurons in rat. Microglial marker expression was increased at transcription as well as translational levels in the substantia nigra (SN) and corpus striatum (CS) of rats exposed to dichlorvos. Activated microglia were seen in SN and CS of dichlorvos-treated animals but were rarely observed in controls. Immunostaining revealed lesser number of TH-positive neurons and higher number of microglia in SN and CS regions after dichlorvos treatment. The mRNA and protein levels of the NADPH oxidase main subunit gp91phox were significantly increased after dichlorvos administration. Dichlorvos exposure also leads to increased level of microglial noxious mediators such as IL-1β, TNF-α and IL-6 in ventral midbrain and CS at transcription as well as translational levels. Data indicate that microglial activation and consequent induction of NADPH oxidase and proinflammatory cytokines such as TNF-α, IL-1β and IL-6 may act as risk factors for Parkinson’s disease by increasing the vulnerability of dopaminergic cells to dichlorvos toxic injury.









Similar content being viewed by others
References
Banati, R. B., Daniel, S. E., & Blunt, S. B. (1998). Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Movement Disorders, 13(2), 221–227.
Barcia, C., de Pablos, V., Bautista-Hernandez, V., Sanchez-Bahillo, A., Bernal, I., & Fernandez-Villalba, E. (2005). Increased plasma levels of TNF-alpha but not of IL1-beta in MPTP-treated monkeys one year after the MPTP administration. Parkinsonism and Related Disorders, 11, 435–439.
Binukumar, B. K., Bal, A., Kandimalla, R. J., & Gill, K. D. (2010). Nigrostriatal neuronal death following chronic dichlorvos exposure: crosstalk between mitochondrial impairments, alpha synuclein aggregation, oxidative damage and behavioral changes. Molecular Brain, 3(1), 35.
Block, M. L., Zecca, L., & Hong, J. S. (2007). Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews Neuroscience, 8, 57–69.
Boka, G., Anglade, P., Wallach, D., Javoy-Agid, F., & Agid, Y. (1994). Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neuroscience Letters, 172, 151–154.
Brodacki, B., Staszewski, J., Toczylowska, B., Kozlowska, E., & Drela, N. (2008). Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neuroscience Letters, 441, 158–162.
Cardona, A. E., Huang, D., Sasse, M. E., & Ransohoff, R. M. (2006). Isolation of murine microglial cells for RNA analysis or flow cytometry. Nature Protocols, 1(4), 1947–1951.
Carvey, P. M., Chen, E. Y., Lipton, J. W., Tong, C. W., Chang, Q. A., & Ling, Z. D. (2005). Intra-parenchymal injection of tumor necrosis factor-alpha and interleukin 1-beta produces dopamine neuron loss in the rat. Journal of Neural Transmission, 112, 601–612.
Chen, H., Jacobs, E., Schwarzschild, M. A., McCullogh, M. L., Calle, E. E., Thun, M. J., et al. (2005). Nonsteroidal anti-inflammatory drug use and the risk for Parkinson’s disease. Annals of Neurology, 58, 963–967.
Fahn, S. (2003). Description of Parkinson’s disease as a clinical syndrome. Annals of the New York Academy of Sciences, 991, 1–14.
Ferger, B., Leng, A., Mura, A., Hengerer, B., & Feldon, J. (2004). Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. Journal of Neurochemistry, 89(4), 822–833.
Ferrer, I., Blanco, R., Carmona, M., Puig, B., & Barrachina, M. (2001). Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson’s disease and Dementia with Lewy bodies. Journal of Neural Transmission, 108, 1383–1396.
Gao, H. M., Liu, B., & Hong, J. S. (2003a). Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. Journal of Neuroscience, 23, 6181–61817.
Gao, H. M., Liu, B., Zhang, W., & Hong, J. S. (2003b). Critical role of microglial NADPH oxidase-derived free radicals in the MPTP model of parkinson’s disease. FASEB Journal, 17(13), 1954–1956.
Gerhard, A., Pavese, N., Hotton, G., Turkheimer, F., & Es, M. (2006). In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiology of Diseases, 21, 404–412.
Green, S. P., Cairns, B., Rae, J., Errett-Baroncini, C., Hongo, J. A., & Erickson, R. W. (2001). Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. Journal of Cerebral Blood Flow and Metabolism, 21, 374–384.
Greenamyre, J. T., MacKenzie, G. M., Peng, T. I., & Stephans, S. E. (1999). Mitochondrial dysfunction in Parkinsonsís disease. Biochemical Society symposium, 66, 85–97.
Hald, A., & Lotharius, J. (2005). Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Experimental Neurology, 193, 279–290.
Hartmann, A., Troadec, J. D., Hunot, S., Kikly, K., & Faucheux, B. A. (2001). Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson’s disease, but pathway inhibition results in neuronal necrosis. Journal of Neuroscience, 21, 2247–2255.
Hasegawa, E., Takeshige, K., Oishi, T., Murai, Y., & Minakami, S. (1990). 1-Methyl-4- phenylpyridinium (MPP) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochemical and Biophysical Research Communications, 170, 1049–1055.
Hunot, S., Dugas, N., Faucheux, B., Hartmann, A., & Tardieu, M. (1999). FcepsilonRII/CD23 is expressed in Parkinson’s disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. Journal of Neuroscience, 19, 3440–3447.
Kaur, P., Radotra, B., Minz, R. W., & Gill, K. D. (2007). Impaired mitochondrial energy metabolism and neuronal apoptotic cell death after chronic dichlorvos (OP) exposure in rat brain. Neurotoxicology, 28, 1208–1219.
Kim, W. G., Mohney, R. P., Wilson, B., Jeohn, G. H., Liu, B., & Hong, J. S. (2000). Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. Journal of Neuroscience, 20, 6309–6316.
Langston, J. W., Forno, L. S., Tetrud, J., Reeves, A. G., Kaplan, J. A., & Karluk, D. (1999). Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine exposure. Annals of Neurology, 46, 598–605.
Lawson, L. J., Perry, V. H., Dri, P., & Gordon, S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 39, 151–170.
Ling, Z., Gayle, D. A., Ma, S. Y., Lipton, J. W., Tong, C. W., & Hong, J. S. (2002). In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Movement Disorders, 17, 116–124.
Liou, H., Tsai, M., Chen, C. J., & Jeng, J. (1997). Environmental risk factors for Parkinson’s disease: a case control study in Taiwan. Neurology, 48, 1583–1588.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Manning-Bog, A. B., McCormack, A. L., Li, J., Uversky, V. N., Fink, A. L., & Di Monte, D. A. (2002). The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. Journal of Biological Chemistry, 277(3), 1641–1644.
Mayeux, R. (2003). Epidemiology of neurodegeneration. Annual Review of Neuroscience, 26, 81–104.
McCormack, A. L., Thiruchelvam, M., Manning-Bog, A. B., Thiffault, C., Langston, J. W., Cory-Slechta, D. A., et al. (2002). Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiology of Diseases, 10(2), 119–127.
McGeer, P. L., Itagaki, S., Boyes, B. E., & McGeer, E. G. (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology, 38, 1285–1291.
McGeer, P. L., & McGeer, E. G. (2004). Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism and Related Disorders, 10(suppl 1), S3–S7.
McGeer, P. L., Yasojima, K., & McGeer, E. G. (2002). Association of interleukin-1 beta polymorphisms with idiopathic Parkinson’s disease. Neuroscience Letters, 326, 67–69.
Mogi, M., Harada, M., Riederer, P., Narabayashi, H., & Fujita, K. (1994). Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neuroscience Letters, 165, 208–210.
Mogi, M., Togari, A., Kondo, T., Mizuno, Y., & Komure, O. (2000). Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. Journal of Neural Transmission, 107, 335–341.
Ouchi, Y., Yoshikawa, E., Sekine, Y., Futatsubashi, M., & Kanno, T. (2005). Microglial activation and dopamine terminal loss in early Parkinson’s disease. Annals of Neurology, 57, 168–175.
Parvathenani, L. K., Tertyshnikova, S., Greco, C. R., Roberts, S. B., Robertson, B., & Posmantur, R. (2003). P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. Journal of Biological Chemistry, 278, 13309–13317.
Paxinos, G., & Watson, C. (1986). The rat brain in stereotaxic coordinates. London: Academic Press.
Priyadarshi, A., Khuder, S. A., Schaub, E. A., & Priyadarshi, S. S. (2001). Environmental risk factors and Parkinson’s disease: a metaanalysis. Environmental Research, 86(2), 122–127.
Rajput, A. H., Uitti, R. J., & Stern, W. (1987). Geography, drinking water chemistry, pesticides and herbicides and the etiology of Parkinson’s disease. Canadian Journal of Neurological Sciences, 14, 414–418.
Rousselet, E., Callebert, J., Parain, K., Joubert, C., Hunot, S., & Hartmann, A. (2002). Role of TNF-alpha receptors in mice intoxicated with the Parkinsonian toxin MPTP. Experimental Neurology, 177, 183–192.
Sawada, M., Imamura, K., & Nagatsu, T. (2006). Role of cytokines in inflammatory process in Parkinson’s disease. Journal of Neural Transmission Supplementum, 70, 373–381.
Schapira, A. H. (2007). Mitochondrial dysfunction in Parkinson’s disease. Cell Death and Differentiation, 14, 1261–1266.
Schulte, T., Schols, L., Muller, T., Woitalla, D., Berger, K., & Kruger, R. (2002). Polymorphisms in the interleukin-1 alpha and beta genes and the risk for Parkinson’s disease. Neuroscience Letters, 326, 70–72.
Semchuk, K. M., Love, E. J., & Lee, R. G. (1992). Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology, 42(7), 1328–1335.
Sherer, T. B., Betarbet, R., Kim, J. H., & Greenamyre, J. T. (2003). Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neuroscience Letters, 341, 87–90.
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., & Goedert, M. (1997). Synuclein in Lewy bodies. Nature, 388, 839–840.
Sriram, K., Matheson, J. M., Benkovic, S. A., Miller, D. B., Luster, M. I., & O’Callaghan, J. P. (2002). Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB Journal, 16, 1474–1476.
Stone, T. W., & Behan, W. M. (2007). Interleukin-1beta but not tumor necrosis factor-alpha potentiates neuronal damage by quinolinic acid: protection by an adenosine A2A receptor antagonist. Journal of Neuroscience Research, 85, 1077–1085.
Teismann, P., & Schulz, J. B. (2004). Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell and Tissue Research, 318, 149–161.
Thiruchelvam, M., McCormack, A., Richfield, E. K., Baggs, R. B., Tank, A. W., Di Monte, D. A., et al. (2003). Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. European Journal of Neuroscience, 18(3), 589–600.
Thiruchelvam, M., Richfield, E. K., Baggs, R. B., Tank, A. W., & Cory-Slechta, D. A. (2000). The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. Journal of Neuroscience, 20(24), 9207–9214.
Thiruchelvam, M., Richfield, E. K., Goodman, B. M., Baggs, R. B., & Cory-Slechta, D. A. (2002). Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology, 23(4–5), 621–633.
Turrens, J. F., & Boveris, A. (1980). Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochemical Journal, 191, 421–427.
Van Den Eeden, S. K., Tanner, C. M., Bernstein, A. L., Fross, R. D., Leimpeter, A., Bloch, D. A., et al. (2003). Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. American Journal of Epidemiology, 157, 1015–1022.
Wooten, G. F. (1997). Neurochemistry and neuropharmacology of Parkinson’s disease. In R. L. Watts & W. C. Koller (Eds.), Movement disorders: neurologic principles and practice (pp. 153–160). New York: McGraw-Hill.
Wu, D. C., Teismann, P., Tieu, K., Vila, M., Jackson-Lewis, V., Ischiropoulos, H., et al. (2003). NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America, 100, 6145–6150.
Acknowledgments
The financial assistance provided to Binukumar B.K. by Indian Council of Medical Research, New Delhi, India, is greatly acknowledged.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Binukumar, B.K., Bal, A. & Gill, K.D. Chronic Dichlorvos Exposure: Microglial Activation, Proinflammatory Cytokines and Damage to Nigrostriatal Dopaminergic System. Neuromol Med 13, 251–265 (2011). https://doi.org/10.1007/s12017-011-8156-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-011-8156-8