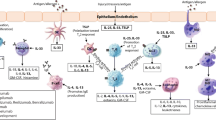
Abstract
Immunoglobulin E (IgE)-mediated food allergy is a real public health problem worldwide. The prevalence of food allergy is particularly high in children. Patients with food allergy experience high morbidity with a change in quality of life due to the risk of severe anaphylaxis. Current treatment options are poor. Allergen avoidance is widely recommended but exposes patients to accidental ingestion. Oral immunotherapy is also used in patients with food allergies to the most common allergens. Oral immunotherapy consists of a daily administration of small, gradually increasing amounts of allergens to induce desensitisation. This procedure aims at inducing immune tolerance to the ingested food allergens. However, some patients experience adverse reactions and discontinue oral immunotherapy.
Given that IgE plays a crucial role in food allergy and anti-IgE are effective in allergic asthma, the use of anti-IgE therapeutic monoclonal antibodies (mAbs) such as omalizumab has been assessed in food allergy patients. The use of omalizumab as a monotherapy in food allergy has not been extensively studied but looks promising. There is more published evidence regarding the effect of omalizumab and oral immunotherapy in food allergy. Given the promising results of oral immunotherapy regarding sustained tolerance in clinical trials and the potential capacity of omalizumab to reduce symptoms in case of accidental exposure, a strategy combining oral immunotherapy with omalizumab pre-treatment has been suggested as a safer option in patients with severe food allergy compared to isolated therapy. Omalizumab seems useful in ensuring safer administration of oral immunotherapy with the oral immunotherapy maintenance dose being reached more rapidly. Quality-of-life improvement is greater with oral immunotherapy + omalizumab compared to oral immunotherapy alone. Moreover, sustained unresponsiveness is achieved more frequently with omalizumab. Considering that precision medicine and personalised therapy are major goals for allergic diseases, predictive biomarkers are crucial in order to identify food allergy patients more likely to benefit from anti-IgE therapies.

Similar content being viewed by others
References
Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, Beyer K, Bindslev-Jensen C, Burks W, Ebisawa M, Eigenmann P, Knol E, Nadeau KC, Poulsen LK, van Ree R, Santos AF, du T G, Dhami S, Nurmatov U, Boloh Y, Makela M, O’Mahony L, Papadopoulos N, Sackesen C, Agache I, Angier E, Halken S, Jutel M, Lau S, Pfaar O, Ryan D, Sturn G, Varga EM, van Wijk RG, Sheikh A, Muraro A (2018) EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 73:799–815. https://doi.org/10.1111/all.13319
Tang ML, Mullins RJ (2017) Food allergy: is prevalence increasing? Intern Med J 47:256–261. https://doi.org/10.1111/imj.13362
Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH (2011) Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Am Acad Dermatol 64:175–192
Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, Sundaram V, Paige NM, Towfigh A, Hulley BJ, Shekelle PG (2010) Diagnosing and managing common food allergies: a systematic review. Jama 303:1848–56. https://doi.org/10.1001/jama.2010.582
Ansotegui IJ, Melioli G, Canonica GW, Caraballo L, Villa E, Ebisawa M, Passalacqua G, Savi E, Ebo D, Gómez RM, Luengo Sánchez O, Oppenheimer JJ, Jensen-Jarolim E, Fischer DA, Haahtela T, Antila M, Bousquet JJ, Cardona V, Chiang WC, Demoly PM, DuBuske LM, Ferrer Puga M, Gerth van Wijk R, González Díaz SN, Gonzalez-Estrada A, Jares E, Kalpaklioğlu AF, Kase Tanno L, Kowalski ML, Ledford DK, Monge Ortega OP, Morais Almeida M, Pfaar O, Poulsen LK, Pawankar R, Renz HE, Romano AG, Rosário Filho NA, Rosenwasser L, Sánchez Borges MA, Scala E, Senna GE, Sisul JC, Tang MLK, Thong BY, Valenta R, Wood RA, Zuberbier T (2020) IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J 13:100080. https://doi.org/10.1016/j.waojou.2019.100080
Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, Dubois AE, Halken S, Hoffmann-Sommergruber K, Poulsen LK, Roberts G, Van Ree R, Vlieg-Boerstra BJ, Sheikh A (2014) The 570 epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy 69:62–75. https://doi.org/10.1111/all.12305
Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A (2014) Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy 69:992–1007. https://doi.org/10.1111/all.12423
Pouessel G, Beaudouin E, Tanno LK, Drouet M, Deschildre A, Labreuche J, Renaudin JM (2019) Food-related anaphylaxis fatalities: analysis of the Allergy Vigilance Network(®) database. Allergy 74:1193–1196. https://doi.org/10.1111/all.13717
Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, Ruëff F, Nemat K, Maris I, Roumpedaki E, Scherer K, Ott H, Reese T, Mustakov T, Lang R, Fernandez-Rivas M, Kowalski ML, Bilò MB, Hourihane JO, Papadopoulos NG, Beyer K, Muraro A, Worm M (2016) Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol 137:1128-1137.e1. https://doi.org/10.1016/j.jaci.2015.11.015
Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ (2017) Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract 5:1169–1178. https://doi.org/10.1016/j.jaip.2017.06.031
Lieberman JA, Sicherer SH (2011) Quality of life in food allergy. Curr Opin Allergy Clin Immunol 11:236–42. https://doi.org/10.1097/ACI.0b013e3283464cf0
Sicherer SH, Noone SA, Muñoz-Furlong A (2001) The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol 87:461–4. https://doi.org/10.1016/s1081-1206(10)62258-2
King RM, Knibb RC, Hourihane JO (2009) Impact of peanut allergy on quality of life, stress and anxiety in the family. Allergy 64:461–8. https://doi.org/10.1111/j.1398-9995.2008.01843.x
Ballmer-Weber BK, Hoffmann-Sommergruber K (2011) Molecular diagnosis of fruit and vegetable allergy. Curr Opin Allergy Clin Immunol 11:229–35. https://doi.org/10.1097/ACI.0b013e3283464c74
Eiwegger T, Hung L, San Diego KE, O’Mahony L, Upton J (2019) Recent developments and highlights in food allergy. Allergy 74:2355–2367. https://doi.org/10.1111/all.14082
Patel G, Saltoun C (2019) Skin testing in allergy. Allergy Asthma Proc 40:366–368. https://doi.org/10.2500/aap.2019.40.4248
Treudler R, Simon JC (2013) Overview of component resolved diagnostics. Curr Allergy Asthma Rep 13:110–7. https://doi.org/10.1007/s11882-012-0318-8
Sicherer SH, Sampson HA (2014) Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 133:291–307; quiz 308. https://doi.org/10.1016/j.jaci.2013.11.020
Beyer K, Grabenhenrich L, Härtl M, Beder A, Kalb B, Ziegert M, Finger A, Harandi N, Schlags R, Gappa M, Puzzo L, Röblitz H, Millner-Uhlemann M, Büsing S, Ott H, Lange L, Niggemann B (2015) Predictive values of component-specific IgE for the outcome of peanut and hazelnut food challenges in children. Allergy 70:90–8. https://doi.org/10.1111/all.12530
Asarnoj A, Nilsson C, Lidholm J, Glaumann S, Östblom E, Hedlin G, van Hage M, Lilja G, Wickman M (2012) Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol 130:468–72. https://doi.org/10.1016/j.jaci.2012.05.019
Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, Melioli G, Nunes C, Passalacqua G, Rosenwasser L, Sampson H, Sastre J, Bousquet J, Zuberbier T; WAO-ARIA-GA2LEN Task Force: Allen K, Asero r, Bohle B, Cox L, de Blay F, Ebisawa M, Maximiliano-Gomez R, Gonzalez-Diaz S , Haahtela T, Holgate S, Jakob T, Larche M, Matricardi PM, Oppenheimer J, Poulsen LK, Renz HE, Rosario N, Rothenberg M, Sanchez-Borges M, Scala E, Valenta R (2013) A WAO - ARIA - GA²LEN consensus document on molecularbased allergy diagnostics. World Allergy Organ J 6(1):17. https://doi.org/10.1186/1939-4551-6-17. PMID: 24090398; PMCID: PMC3874689
Sampson HA (2001) Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 107:891–6. https://doi.org/10.1067/mai.2001.114708
Roberts G, Lack G (2005) Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol 115:1291–6. https://doi.org/10.1016/j.jaci.2005.02.038
Tuano KS, Davis CM (2015) Utility of component-resolved diagnostics in food allergy. Curr Allergy Asthma Rep 15:32. https://doi.org/10.1007/s11882-015-0534-0
Lawrence MG, Woodfolk JA, Schuyler AJ, Stillman LC, Chapman MD, Platts-Mills TA (2017) Half-life of IgE in serum and skin: consequences for anti-IgE therapy in patients with allergic disease. J Allergy Clin Immunol 139:422-428.e4. https://doi.org/10.1016/j.jaci.2016.04.056
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG (2009) Follicular helper T cells: lineage and location. Immunity 30:324–35. https://doi.org/10.1016/j.immuni.2009.03.003
Akdis CA, Arkwright PD, Bruggen MC, Busse W, Gadina M, Guttman-Yassky E, Kabashima K, Mitamura Y, Vian L, Wu J, Palomares O (2020) Type 2 immunity in the skin and lungs. Allergy 75:1582–1605. https://doi.org/10.1111/all.14318
Walker JA, McKenzie ANJ (2018) TH2 cell development and function. Nat Rev Immunol 18:121–133. https://doi.org/10.1038/nri.2017.118
Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, Tumes DJ, Okamoto Y (2017) Th2 cells in health and disease. Annu Rev Immunol 35:53–84. https://doi.org/10.1146/annurev-immunol-051116-052350
Reber LL, Hernandez JD, Galli SJ (2017) The pathophysiology of anaphylaxis. J Allergy Clin Immunol 140:335–348. https://doi.org/10.1016/j.jaci.2017.06.003
Galli SJ, Gaudenzio N, Tsai M (2020) Mast cells in inflammation and disease: recent progress and ongoing concerns. Annu Rev Immunol 38:49–77. https://doi.org/10.1146/annurev-immunol-071719-094903
Blank U, Charles N, Benhamou M (2016) The high-affinity immunoglobulin E receptor as pharmacological target. Eur J Pharmacol 778:24–32. https://doi.org/10.1016/j.ejphar.2015.05.070
Golberg L (1978) Toxicology: has a new era dawned? Pharmacol Rev 30:351–70
McDonnell JM, Calvert R, Beavil RL, Beavil AJ, Henry AJ, Sutton BJ, Gould HJ, Cowburn D (2001) The structure of the IgE Cepsilon2 domain and its role in stabilizing the complex with its high-affinity receptor FcepsilonRIalpha. Nat Struct Biol 8:437–41. https://doi.org/10.1038/87603
Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, Jardieu PM (1993) Humanization of an antibody directed against IgE. J Immunol 151:2623–32
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA (2011) Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 364:1005–15. https://doi.org/10.1056/NEJMoa1009705
Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, Pföhler C, Staubach P, Treudler R, Wedi B, Magerl M (2018) Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J Allergy Clin Immunol 141:638–649. https://doi.org/10.1016/j.jaci.2017.06.032
Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M (2017) Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J Allergy Clin Immunol 139:1772-1781.e1. https://doi.org/10.1016/j.jaci.2016.08.050
Wright JD, Chu HM, Huang CH, Ma C, Chang TW, Lim C (2015) Structural and physical basis for anti-IgE therapy. Sci Rep 5:11581. https://doi.org/10.1038/srep11581
Davies AM, Allan EG, Keeble AH, Delgado J, Cossins BP, Mitropoulou AN, Pang MOY, Ceska T, Beavil AJ, Craggs G, Westwood M, Henry AJ, McDonnell JM, Sutton BJ (2017) Allosteric mechanism of action of the therapeutic anti-IgE antibody omalizumab. J Biol Chem 292:9975–9987. https://doi.org/10.1074/jbc.M117.776476
Pennington LF, Tarchevskaya S, Brigger D, Sathiyamoorthy K, Graham MT, Nadeau KC, Eggel A, Jardetzky TS (2016) Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nat Commun 7:11610. https://doi.org/10.1038/ncomms11610
Chang TW, Davis FM, Sun NC, Sun CR, MacGlashan DW, Hamilton RG Jr (1990) Monoclonal antibodies specific for human IgE-producing B cells: a potential therapeutic for IgE-mediated allergic diseases. Biotechnology (N Y) 8:122–6. https://doi.org/10.1038/nbt0290-122
First biologic for allergy-related asthma (2003 Sep-Oct) FDA Consum 37(5):5. PMID: 14666890
Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, Beeh KM, Ramos S, Canonica GW, Hedgecock S, Fox H, Blogg M, Surrey K (2005) Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 60:309–16. https://doi.org/10.1111/j.1398-9995.2004.00772.x
Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, Owen CE, Jones I, Lowe PJ (2014) Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy 44:1371–85. https://doi.org/10.1111/cea.12400
Gasser P, Tarchevskaya SS, Guntern P, Brigger D, Ruppli R, Zbaren N, Kleinboelting S, Heusser C, Jardetzky TS, Eggel A (2020) The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun 11:165. https://doi.org/10.1038/s41467-019-13815-w
Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, Bernstein JA, Brehler R, Chu CY, Chung WH, Danilycheva I, Grattan C, Hébert J, Katelaris C, Makris M, Meshkova R, Savic S, Sinclair R, Sitz K, Staubach P, Wedi B, Löffler J, Barve A, Kobayashi K, Hua E, Severin T, Janocha R (2019) Ligelizumab for chronic spontaneous urticaria. N Engl J Med 381:1321–1332. https://doi.org/10.1056/NEJMoa1900408
Novartis ligelizumab (QGE031) receives FDA Breakthrough Therapy designation for patients with chronic spontaneous urticaria (CSU) (n.d.) Novartis. https://www.novartis.com/news/media-releases/novartis-ligelizumabqge031-receives-fda-breakthrough-therapy-designation-patients-chronic-spontaneous-urticaria-csu (accessed September 21, 2021)
Vickery BP, Chin S, Burks AW (2011) Pathophysiology of food allergy. Pediatr Clin North Am 58:363–76, ix–x. https://doi.org/10.1016/j.pcl.2011.02.012
Mankad VS, Burks AW (2005) Omalizumab : other indications and unanswered questions. Clin Rev Allergy Immunol 29:17–30. https://doi.org/10.1385/criai:29:1:017
Muraro A, Roberts G, Worm M, Bilo MB, Brockow K, Fernandez Rivas M, Santos AF, Zolkipli ZQ, Bellou A, Beyer K, Bindslev-Jensen C, Cardona V, Clark AT, Demoly P, Dubois AE, DunnGalvin A, Eigenmann P, Halken S, Harada L, Lack G, Jutel M, Niggemann B, Rueff F, Timmermans F, Vlieg-Boerstra BJ, Werfel T, Dhami S, Panesar S, Akdis CA, Sheikh A (2014) Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 69:1026–45. https://doi.org/10.1111/all.12437
Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, Cardona V, Dubois A, duToit G, Eigenmann P, Fernandez Rivas M, Halken S, Hickstein L, Host A, Knol E, Lack G, Marchisotto MJ, Niggemann B, Nwaru BI, Papadopoulos NG, Poulsen LK, Santos AF, Skypala I, Schoepfer A, Van Ree R, Venter C, Worm M, Vlieg-Boerstra B, Panesar S, de Silva D, Soares-Weiser K, Sheikh A, Ballmer-Weber BK, Nilsson C, de Jong NW, Akdis CA (2014) EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 69:1008–25. https://doi.org/10.1111/all.12429
Muraro A, Agache I, Clark A, Sheikh A, Roberts G, Akdis CA, Borrego LM, Higgs J, Hourihane JO, Jorgensen P, Mazon A, Parmigiani D, Said M, Schnadt S, van Os-Medendorp H, Vlieg-Boerstra BJ, Wickman M (2014) EAACI food allergy and anaphylaxis guidelines: managing patients with food allergy in the community. Allergy 69:1046–57. https://doi.org/10.1111/all.12441
Muraro A, Hoffmann-Sommergruber K, Holzhauser T, Poulsen LK, Gowland MH, Akdis CA, Mills EN, Papadopoulos N, Roberts G, Schnadt S, van Ree R, Sheikh A, Vieths S (2014) EAACI Food Allergy and Anaphylaxis Guidelines. Protecting consumers with food allergies: understanding food consumption, meeting regulations and identifying unmet needs. Allergy 69:1464–72. https://doi.org/10.1111/all.12453
Costa C, Coimbra A, Vitor A, Aguiar R, Ferreira AL, Todo-Bom A (2020) Food allergy-From food avoidance to active treatment. Scand J Immunol 91:e12824. https://doi.org/10.1111/sji.12824
Bollinger ME, Dahlquist LM, Mudd K, Sonntag C, Dillinger L, McKenna K (2006) The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol 96:415–21. https://doi.org/10.1016/s1081-1206(10)60908-8
Bilaver LA, Chadha AS, Doshi P, O’Dwyer L, Gupta RS (2019) Economic burden of food allergy: a systematic review. Ann Allergy Asthma Immunol 122:373-380.e1. https://doi.org/10.1016/j.anai.2019.01.014
Scurlock AM, Vickery BP, Hourihane JO, Burks AW (2010) Pediatric food allergy and mucosal tolerance. Mucosal Immunol 3:345–54. https://doi.org/10.1038/mi.2010.21
Hussey Freeland DM, Fan-Minogue H, Spergel JM, Chatila TA, Nadeau KC (2016) Advances in food allergy oral immunotherapy: toward tolerance. Curr Opin Immunol 42:119–123. https://doi.org/10.1016/j.coi.2016.08.002
Burks AW, Sampson HA, Plaut M, Lack G, Akdis CA (2018) Treatment for food allergy. J Allergy Clin Immunol 141:1–9. https://doi.org/10.1016/j.jaci.2017.11.004
Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, Hiegel A, Kamilaris J, Carlisle S, Yue X, Kulis M, Pons L, Vickery B, Burks AW (2011) A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol 127:654–60. https://doi.org/10.1016/j.jaci.2010.12.1111
Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, Stablein D, Henning AK, Vickery BP, Liu AH, Scurlock AM, Shreffler WG, Plaut M, Sampson HA (2012) Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 367:233–43. https://doi.org/10.1056/NEJMoa1200435
Leung DY, Sampson HA, Yunginger JW, Burks AW Jr, Schneider LC, Wortel CH, Davis FM, Hyun JD, Shanahan WR Jr (2003) Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 348:986–93. https://doi.org/10.1056/NEJMoa022613
Kolbinger F, Saldanha J, Hardman N, Bendig MM (1993) Humanization of a mouse anti-human IgE antibody: a potential therapeutic for IgE-mediated allergies. Protein Eng 6:971–80. https://doi.org/10.1093/protein/6.8.971
Chang TW (2000) The pharmacological basis of anti-IgE therapy. Nat Biotechnol 18:157–62. https://doi.org/10.1038/72601
Rafi A, Do LT, Katz R, Sheinkopf LE, Simons CW, Klaustermeyer W (2010) Effects of omalizumab in patients with food allergy. Allergy Asthma Proc 31:76–83. https://doi.org/10.2500/aap.2010.31.3304
Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, Wong DA (2011) A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol 127:1309–10.e1. https://doi.org/10.1016/j.jaci.2011.01.051
Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT (2012) Oral immunotherapy and anti-IgE antibody-adjunctive treatment for food allergy. Immunol Allergy Clin North Am 32:111–33. https://doi.org/10.1016/j.iac.2011.11.004
Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT (2013) A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol 132:1368–74. https://doi.org/10.1016/j.jaci.2013.09.046
MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, Heimall J, Makhija M, Robison R, Chinthrajah RS, Lee J, Lebovidge J, Dominguez T, Rooney C, Lewis MO, Koss J, Burke-Roberts E, Chin K, Logvinenko T, Pongracic JA, Umetsu DT, Spergel J, Nadeau KC, Schneider LC (2017) Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 139:873-881.e8. https://doi.org/10.1016/j.jaci.2016.08.010
Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT (2011) Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol 127:1622–4. https://doi.org/10.1016/j.jaci.2011.04.009
Takahashi M, Soejima K, Taniuchi S, Hatano Y, Yamanouchi S, Ishikawa H, Irahara M, Sasaki Y, Kido H, Kaneko K (2017) Oral immunotherapy combined with omalizumab for high-risk cow’s milk allergy: a randomized controlled trial. Sci Rep 7:17453. https://doi.org/10.1038/s41598-017-16730-6
Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, Plaut M, Sampson HA (2016) A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 137:1103–1110. https://doi.org/10.1016/j.jaci.2015.10.005
Begin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, Trela A, Tavassoli M, Hoyte E, O’Riordan G, Blakemore A, Seki S, Hamilton RG, Nadeau KC (2014) Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol 10:7. https://doi.org/10.1186/1710-1492-10-7
Andorf S, Manohar M, Dominguez T, Bloc W, Tupa D, Kshirsagar RA, Sampath V, Chinthrajah RS, Nadeau KC (2017) Observational long-term follow-up study of rapid food oral immunotherapy with omalizumab. Allergy Asthma Clin Immunol 13:51. https://doi.org/10.1186/s13223-017-0223-8
Otani IM, Begin P, Kearney C, Dominguez TL, Mehrotra A, Bacal LR, Wilson S, Nadeau K (2014) Multiple-allergen oral immunotherapy improves quality of life in caregivers of food-allergic pediatric subjects. Allergy Asthma Clin Immunol 10:25. https://doi.org/10.1186/1710-1492-10-25
Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, Rudman Spergel A, Desai M, Galli SJ, Nadeau KC, Chinthrajah RS (2018) Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol 3:85–94. https://doi.org/10.1016/s2468-1253(17)30392-8
Manohar M, Dunham D, Gupta s, Yan Z, Zhang W, Minnicozzi S, Kirkey M, Bunning B, Chowdhury RR, Galli SJ, Boyd SD, Kost LE, Chinthrajah RS, Desai M, Oettgen HC, Maecker HT, Yu W, DeKruyff RH, Andorf S, Nadeau KC (2021) Immune changes beyond Th2 pathways during rapid multifood immunotherapy enabled with omalizumab. Allergy. https://doi.org/10.1111/all.14833
Andorf S, Purington N, Kumar D, Long A, O’Laughlin KL, Sicherer S, Sampson H, Cianferoni A, Whitehorn TB, Petroni D, Makhija M, Robison RG, Lierl M, Logsdon S, Desai M, Galli SJ, Rael E, Assa’ad A, Chinthrajah S, Pongracic J, Spergel JM, Tam J, Tilles S, Wang J, Nadeau K (2019) A phase 2 randomized controlled multisite study using omalizumab-facilitated rapid desensitization to test continued vs discontinued dosing in multifood allergic individuals. E Clinical Medicine 7:27–38. https://doi.org/10.1016/j.eclinm.2018.12.006
Lafuente I, Mazon A, Nieto M, Uixera S, Pina R, Nieto A (2014) Possible recurrence of symptoms after discontinuation of omalizumab in anti-IgE-assisted desensitization to egg. Pediatr Allergy Immunol 25:717–9. https://doi.org/10.1111/pai.12259
Martorell-Calatayud C, Michavila-Gomez A, Martorell-Aragones A, Molini-Menchon N, Cerda-Mir JC, Felix-Toledo R, De Las Marinas-Alvarez MD (2016) Anti-IgE-assisted desensitization to egg and cow’s milk in patients refractory to conventional oral immunotherapy. Pediatr Allergy Immunol 27:544–6. https://doi.org/10.1111/pai.12567
Yun J, Katelaris CH (2009) Food allergy in adolescents and adults. Intern Med J 39:475–8. https://doi.org/10.1111/j.1445-5994.2009.01967.x
McGowan EC, Savage JH, Courneya JP, Sterba PM, Parihar S, Lin J, Gimenez G, Sampson HA, Schroeder J, MacGlashan D, Wood RA, Hamilton RG, Saini S (2014) Relationship of IgE to basophil phenotypes in peanut-sensitized adults. J Allergy Clin Immunol 134:746–749. https://doi.org/10.1016/j.jaci.2014.04.040
Takahashi M, Taniuchi S, Soejima K, Hatano Y, Yamanouchi S, Kaneko K (2015) Successful desensitization in a boy with severe cow’s milk allergy by a combination therapy using omalizumab and rush oral immunotherapy. Allergy Asthma Clin Immunol 11:18. https://doi.org/10.1186/s13223-015-0084-y
Stranks AJ, Minnicozzi SC, Miller SJ, Burton OT, Logsdon SL, Spergel JM, Nadeau KC, Pongracic JA, Umetsu DT, Rachid R, MacGinnitie AJ, Schneider LC, Oettgen HC (2019) Immunoglobulin E blockade during food allergen ingestion enhances the induction of inhibitory immunoglobulin G antibodies. Ann Allergy Asthma Immunol 122:213–215. https://doi.org/10.1016/j.anai.2018.10.024
Brandstrom J, Vetander M, Sundqvist AC, Lilja G, Johansson SGO, Melen E, Sverremark-Ekstrom E, Nopp A, Nilsson C (2019) Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents. Clin Exp Allergy 49:1328–1341. https://doi.org/10.1111/cea.13469
Nopp A, Johansson SG, Ankerst J, Bylin G, Cardell LO, Grönneberg R, Irander K, Palmqvist M, Oman H (2006) Basophil allergen threshold sensitivity: a useful approach to anti-IgE treatment efficacy evaluation. Allergy 61:298–302. https://doi.org/10.1111/j.1398-9995.2006.00987.x
Suarez-Farinas M, Suprun M, Chang HL, Gimenez G, Grishina G, Getts R, Nadeau K, Wood RA, Sampson HA (2019) Predicting development of sustained unresponsiveness to milk oral immunotherapy using epitope-specific antibody binding profiles. J Allergy Clin Immunol 143:1038–1046. https://doi.org/10.1016/j.jaci.2018.10.028
Siracusa MC, Kim BS, Spergel JM, Artis D (2013) Basophils and allergic inflammation. J Allergy Clin Immunol 132:789–801; quiz 788. https://doi.org/10.1016/j.jaci.2013.07.046
MacGlashan DW Jr, Savage JH, Wood RA, Saini SS (2012) Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol 130:1130-1135.e5. https://doi.org/10.1016/j.jaci.2012.05.038
Gernez Y, Tirouvanziam R, Yu G, Ghosn EE, Reshamwala N, Nguyen T, Tsai M, Galli SJ, Herzenberg LA, Herzenberg LA, Nadeau KC (2011) Basophil CD203c levels are increased at baseline and can be used to monitor omalizumab treatment in subjects with nut allergy. Int Arch Allergy Immunol 154:318–27. https://doi.org/10.1159/000321824
Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA (2012) Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol 130:1123-1129.e2. https://doi.org/10.1016/j.jaci.2012.05.039
Nilsson C, Nordvall L, Johansson SG, Nopp A (2014) Successful management of severe cow’s milk allergy with omalizumab treatment and CD-sens monitoring. Asia Pac Allergy 4:257–60. https://doi.org/10.5415/apallergy.2014.4.4.257
Brandstrom J, Vetander M, Lilja G, Johansson SG, Sundqvist AC, Kalm F, Nilsson C, Nopp A (2017) Individually dosed omalizumab: an effective treatment for severe peanut allergy. Clin Exp Allergy 47:540–550. https://doi.org/10.1111/cea.12862
Frischmeyer-Guerrerio PA, Masilamani M, Gu W, Brittain E, Wood R, Kim J, Nadeau K, Jarvinen KM, Grishin A, Lindblad R, Sampson HA (2017) Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol 140:1043-1053.e8. https://doi.org/10.1016/j.jaci.2017.03.028
Bedoret D, Singh AK, Shaw V, Hoyte EG, Hamilton R, DeKruyff RH, Schneider LC, Nadeau KC, Umetsu DT (2012) Changes in antigen-specific T-cell number and function during oral desensitization in cow’s milk allergy enabled with omalizumab. Mucosal Immunol 5:267–76. https://doi.org/10.1038/mi.2012.5
Abdel-Gadir A, Schneider L, Casini A, Charbonnier LM, Little SV, Harrington T, Umetsu DT, Rachid R, Chatila TA (2018) Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin Exp Allergy 48:825–836. https://doi.org/10.1111/cea.13161
Galli SJ (2016) Toward precision medicine and health: opportunities and challenges in allergic diseases. J Allergy Clin Immunol 137:1289–300. https://doi.org/10.1016/j.jaci.2016.03.006
Golebski K, Layhadi JA, Sahiner U, Steveling-Klein EH, Lenormand MM, Li RCY, Bal SM, Heesters BA, Vilà-Nadal G, Hunewald O, Montamat G, He FQ, Ollert M, Fedina O, Lao-Araya M, Vijverberg SJH, Maitland-van der Zee AH, van Drunen CM, Fokkens WJ, Durham SR, Spits H, Shamji MH (2021) Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 54:291-307.e7. https://doi.org/10.1016/j.immuni.2020.12.013
Licari A, Castagnoli R, Marseglia A, Olivero F, Votto M, Ciprandi G, Marseglia GL (2020) Dupilumab to treat type 2 inflammatory diseases in children and adolescents. Paediatr Drugs 22:295–310. https://doi.org/10.1007/s40272-020-00387-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
L.G. has been an investigator in clinical trials for AstraZeneca, MSD, and Novartis and reports grants or fees for consulting from AstraZeneca, GlaxoSmithKline, Novartis, and Sanofi-Regeneron and fees for consulting from Bayer, Chiesi, MSD, not related to the submitted work. M.M. declares no disclosure of interest. L.L.R. reports grants or consulting fees from Neovacs S.A. not related to the work submitted, and patents issued or pending relating to allergy diagnosis or therapy: 63/079,686, PCT/EP2021/060829, WO2019197607 (A1) and WO2019228674 (A1).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guilleminault, L., Michelet, M. & Reber, L.L. Combining Anti-IgE Monoclonal Antibodies and Oral Immunotherapy for the Treatment of Food Allergy. Clinic Rev Allerg Immunol 62, 216–231 (2022). https://doi.org/10.1007/s12016-021-08902-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-021-08902-0