Abstract
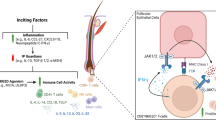
Alopecia areata (AA) is a common chronic tissue-specific autoimmune disease, resulting in hair loss, that affects up to 2% of the general population. The exact pathobiology of AA has still remained elusive, while the common theory is the collapse of the immune privilege of the hair follicle caused by immunological mechanism. Multiple genetic and environment factors contribute to the pathogenesis of AA. There are several clinical treatments for AA, varying from one or multiple well-defined patches to more diffuse or total hair loss of the scalp (alopecia totalis) or hair loss of the entire body (alopecia universalis). The available treatments for AA, such as corticosteroids and other immunomodulators, minoxidil, and contact immunotherapy, are of limited efficacy with a high risk of adverse effects and high recurrence rates, especially for patients with severe AA. Recent insights into the pathogenesis of AA have led to the development of new treatment strategies, such as Janus kinase (JAK) inhibitors, biologics, and several small molecular agents. In addition, modern therapies for AA, including antihistamines, platelet-rich plasma (PRP) injection, and other novel therapies have been well explored. In this review, we discussed the recent advances in the pathogenesis, diagnosis, and treatment of AA.



Similar content being viewed by others
Abbreviations
- AA:
-
Alopecia areata
- AAI:
-
Alopecia areata incognita
- AA-IGA™:
-
AA investigator global assessment
- AD:
-
Atopic dermatitis
- ADTA:
-
Acute diffuse and total alopecia
- AT:
-
Alopecia totalis
- AU:
-
Alopecia universalis
- α-MSH:
-
α-Melanocyte-stimulating hormone
- cAMP:
-
Cyclic adenosine monophosphate
- CI:
-
Contact immunotherapy
- CNV:
-
Copy number variant
- CTLA4:
-
Cytotoxic T lymphocyte–associated antigen 4
- CXCL:
-
Chemokine ligand
- DPCP:
-
Diphenylcyclopropenone
- ERK:
-
Extracellular signal–regulated kinase
- GWAS:
-
Genome-wide association studies
- HF:
-
Hair follicle
- HLA:
-
Human leukocyte antigen
- IC:
-
Intralesional corticosteroids
- IFN:
-
Interferon
- IL:
-
Interleukin
- IP:
-
Immune privileged
- JAK:
-
Janus kinase
- JAKi:
-
JAK inhibitors
- MHC:
-
Major histocompatibility complex
- MICA:
-
MHC class I–related chain A
- NK:
-
Natural killer
- PBMC:
-
Peripheral blood mononuclear cell
- PCT:
-
Pulse corticosteroid therapy
- PDE:
-
Phosphodiesterase
- PRP:
-
Platelet-rich plasma
- RCT:
-
Randomized controlled trial
- ROS:
-
Reactive oxygen species
- SADBE:
-
Squaric acid dibutyl ester
- SALT:
-
Severity of alopecia tool
- SOD:
-
Superoxide dismutase
- STATs:
-
Signal transducers and activators of transcription
- TA:
-
Triamcinolone acetonide
- TC:
-
Topical corticosteroids
- TGF:
-
Transforming growth factor
- TNF:
-
Tumor necrosis factor
- Tregs:
-
T-regulatory cells
- TYK2:
-
Tyrosine kinase 2
- ULBP:
-
UL16-binding protein
- VIP:
-
Vasoactive intestinal peptide
References
Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP (2017) Alopecia areata. Nat Rev Dis Primers 3(1). https://doi.org/10.1038/nrdp.2017.11
Toussi A, Barton VR, Le ST, Agbai ON, Kiuru M (2020) Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.06.047
Lee HH, Gwillim E, Patel KR, Hua T, Rastogi S, Ibler E, Silverberg JI (2020) Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol 82(3):675–682. https://doi.org/10.1016/j.jaad.2019.08.032
Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, Shapiro J (2018) Alopecia areata disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol 78(1):1–12. https://doi.org/10.1016/j.jaad.2017.04.1141
Pourang A, Mesinkovska NA (2020) New and emerging therapies for alopecia areata. Drugs 80(7):635–646. https://doi.org/10.1007/s40265-020-01293-0
Broadley D, McElwee KJ (2020) A “hair-raising” history of alopecia areata. Exp Dermatol 29(3):208–222. https://doi.org/10.1111/exd.14073
Simakou T, Butcher JP, Reid S, Henriquez FL (2019) Alopecia areata: a multifactorial autoimmune condition. J Autoimmun 98:74–85. https://doi.org/10.1016/j.jaut.2018.12.001
Rajabi F, Drake LA, Senna MM, Rezaei N (2018) Alopecia areata: a review of disease pathogenesis. Br J Dermatol 179(5):1033–1048. https://doi.org/10.1111/bjd.16808
Gilhar A, Laufer-Britva R, Keren A, Paus R (2019) Frontiers in alopecia areata pathobiology research. J Allergy Clin Immunol 144(6):1478–1489. https://doi.org/10.1016/j.jaci.2019.08.035
Phan K, Sebaratnam DF (2019) JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 33(5):850–856. https://doi.org/10.1111/jdv.15489
Wang ECE, Dai Z, Christiano AM (2018) Novel therapies for alopecia areata: the era of rational drug development. J Allergy Clin Immunol 141(2):499–504. https://doi.org/10.1016/j.jaci.2017.10.028
Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, Shapiro J (2018) Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol 78(1):15–24. https://doi.org/10.1016/j.jaad.2017.04.1142
Blaumeiser B, van der Goot I, Fimmers R, Hanneken S, Ritzmann S, Seymons K, Betz RC, Ruzicka T, Wienker TF, De Weert J, Lambert J, Kruse R, Nothen MM (2006) Familial aggregation of alopecia areata. J Am Acad Dermatol 54(4):627–632. https://doi.org/10.1016/j.jaad.2005.12.007
Jackow C, Puffer N, Hordinsky M, Nelson J, Tarrand J, Duvic M (1998) Alopecia areata and cytomegalovirus infection in twins: genes versus environment? J Am Acad Dermatol 38(3):418–425. https://doi.org/10.1016/s0190-9622(98)70499-2
Rodriguez TA, Fernandes KE, Dresser KL, Duvic M, National Alopecia Areata R (2010) Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol 62(3):525–527. https://doi.org/10.1016/j.jaad.2009.02.006
Martinez-Mir A, Zlotogorski A, Gordon D, Petukhova L, Mo J, Gilliam TC, Londono D, Haynes C, Ott J, Hordinsky M, Nanova K, Norris D, Price V, Duvic M, Christiano AM (2007) Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet 80(2):316–328. https://doi.org/10.1086/511442
Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, Kim H, Singh P, Lee A, Chen WV, Meyer KC, Paus R, Jahoda CA, Amos CI, Gregersen PK, Christiano AM (2010) Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 466(7302):113–117. https://doi.org/10.1038/nature09114
Betz RC, Petukhova L, Ripke S, Huang H, Menelaou A, Redler S, Becker T, Heilmann S, Yamany T, Duvic M, Hordinsky M, Norris D, Price VH, Mackay-Wiggan J, de Jong A, DeStefano GM, Moebus S, Böhm M, Blume-Peytavi U, Wolff H, Lutz G, Kruse R, Bian L, Amos CI, Lee A, Gregersen PK, Blaumeiser B, Altshuler D, Clynes R, de Bakker PIW, Nöthen MM, Daly MJ, Christiano AM (2015) Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun 6(1). https://doi.org/10.1038/ncomms6966
Petukhova L, Christiano AM (2016) Functional interpretation of genome-wide association study evidence in alopecia areata. J Invest Dermatol 136(1):314–317. https://doi.org/10.1038/JID.2015.402
Jagielska D, Redler S, Brockschmidt FF, Herold C, Pasternack SM, Garcia Bartels N, Hanneken S, Eigelshoven S, Refke M, Barth S, Giehl KA, Kruse R, Lutz G, Wolff H, Blaumeiser B, Bohm M, Blume-Peytavi U, Becker T, Nothen MM, Betz RC (2012) Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol 132(9):2192–2197. https://doi.org/10.1038/jid.2012.129
Oka A, Takagi A, Komiyama E, Yoshihara N, Mano S, Hosomichi K, Suzuki S, Haida Y, Motosugi N, Hatanaka T, Kimura M, Ueda MT, Nakagawa S, Miura H, Ohtsuka M, Tanaka M, Komiyama T, Otomo A, Hadano S, Mabuchi T, Beck S, Inoko H, Ikeda S (2020) Alopecia areata susceptibility variant in MHC region impacts expressions of genes contributing to hair keratinization and is involved in hair loss. EBioMedicine 57:102810. https://doi.org/10.1016/j.ebiom.2020.102810
Petukhova L (2020) An imperative need for further genetic studies of alopecia areata. J Investig Dermatol Symp Proc 20(1):S22–S27. https://doi.org/10.1016/j.jisp.2020.04.003
Fischer J, Degenhardt F, Hofmann A, Redler S, Basmanav FB, Heilmann-Heimbach S, Hanneken S, Giehl KA, Wolff H, Moebus S, Kruse R, Lutz G, Blaumeiser B, Bohm M, Garcia Bartels N, Blume-Peytavi U, Petukhova L, Christiano AM, Nothen MM, Betz RC (2017) Genomewide analysis of copy number variants in alopecia areata in a Central European cohort reveals association with MCHR2. Exp Dermatol 26(6):536–541. https://doi.org/10.1111/exd.13123
Petukhova L, Patel AV, Rigo RK, Bian L, Verbitsky M, Sanna-Cherchi S, Erjavec SO, Abdelaziz AR, Cerise JE, Jabbari A, Christiano AM (2020) Integrative analysis of rare copy number variants and gene expression data in alopecia areata implicates an aetiological role for autophagy. Exp Dermatol 29(3):243–253. https://doi.org/10.1111/exd.13986
Bertolini M, McElwee K, Gilhar A, Bulfone-Paus S, Paus R (2020) Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol 29(8):703–725. https://doi.org/10.1111/exd.14155
Gilhar A, Etzioni A, Paus R (2012) Alopecia areata. N Engl J Med 366(16):1515–1525. https://doi.org/10.1056/NEJMra1103442
Gilhar A, Schrum AG, Etzioni A, Waldmann H, Paus R (2016) Alopecia areata: animal models illuminate autoimmune pathogenesis and novel immunotherapeutic strategies. Autoimmun Rev 15(7):726–735. https://doi.org/10.1016/j.autrev.2016.03.008
Freyschmidt-Paul P, McElwee KJ, Hoffmann R, Sundberg JP, Vitacolonna M, Kissling S, Zoller M (2006) Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol 155(3):515–521. https://doi.org/10.1111/j.1365-2133.2006.07377.x
Gilhar A, Kam Y, Assy B, Kalish RS (2005) Alopecia areata induced in C3H/HeJ mice by interferon-gamma: evidence for loss of immune privilege. J Invest Dermatol 124(1):288–289. https://doi.org/10.1111/j.0022-202X.2004.23580.x
Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, de Jong A, Harel S, DeStefano GM, Rothman L, Singh P, Petukhova L, Mackay-Wiggan J, Christiano AM, Clynes R (2014) Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 20(9):1043–1049. https://doi.org/10.1038/nm.3645
Divito SJ, Kupper TS (2014) Inhibiting Janus kinases to treat alopecia areata. Nat Med 20(9):989–990. https://doi.org/10.1038/nm.3685
Ghraieb A, Keren A, Ginzburg A, Ullmann Y, Schrum AG, Paus R, Gilhar A (2018) iNKT cells ameliorate human autoimmunity: lessons from alopecia areata. J Autoimmun 91:61–72. https://doi.org/10.1016/j.jaut.2018.04.001
Loh SH, Moon HN, Lew BL, Sim WY (2018) Role of T helper 17 cells and T regulatory cells in alopecia areata: comparison of lesion and serum cytokine between controls and patients. J Eur Acad Dermatol Venereol 32(6):1028–1033. https://doi.org/10.1111/jdv.14775
Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, Lacour JP, Passeron T (2014) Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol 150(7):748–751. https://doi.org/10.1001/jamadermatol.2014.504
Tanemura A, Oiso N, Nakano M, Itoi S, Kawada A, Katayama I (2013) Alopecia areata: infiltration of Th17 cells in the dermis, particularly around hair follicles. Dermatology 226(4):333–336. https://doi.org/10.1159/000350933
Han YM, Sheng YY, Xu F, Qi SS, Liu XJ, Hu RM, Miao Y, Huang GQ, Yang QP (2015) Imbalance of T-helper 17 and regulatory T cells in patients with alopecia areata. J Dermatol 42(10):981–988. https://doi.org/10.1111/1346-8138.12978
Abou Rahal J, Kurban M, Kibbi AG, Abbas O (2016) Plasmacytoid dendritic cells in alopecia areata: missing link? J Eur Acad Dermatol Venereol 30(1):119–123. https://doi.org/10.1111/jdv.12932
Ito T, Suzuki T, Sakabe JI, Funakoshi A, Fujiyama T, Tokura Y (2020) Plasmacytoid dendritic cells as a possible key player to initiate alopecia areata in the C3H/HeJ mouse. Allergol Int 69(1):121–131. https://doi.org/10.1016/j.alit.2019.07.009
Bertolini M, Zilio F, Rossi A, Kleditzsch P, Emelianov VE, Gilhar A, Keren A, Meyer KC, Wang E, Funk W, McElwee K, Paus R (2014) Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS One 9(5):e94260. https://doi.org/10.1371/journal.pone.0094260
Zhang X, Zhao Y, Ye Y, Li S, Qi S, Yang Y, Cao H, Yang J, Zhang X (2015) Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Arch Dermatol Res 307(4):319–331. https://doi.org/10.1007/s00403-015-1539-1
Ito T, Kageyama R, Nakazawa S, Honda T (2020) Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp Dermatol 29(8):726–732. https://doi.org/10.1111/exd.14129
Yoon TY, Lee DY, Kim YJ, Lee JY, Kim MK (2014) Diagnostic usefulness of a peribulbar eosinophilic infiltrate in alopecia areata. JAMA Dermatol 150(9):952–956. https://doi.org/10.1001/jamadermatol.2014.62
Wang EHC, Yu M, Breitkopf T, Akhoundsadegh N, Wang X, Shi FT, Leung G, Dutz JP, Shapiro J, McElwee KJ (2016) Identification of autoantigen epitopes in alopecia areata. J Invest Dermatol 136(8):1617–1626. https://doi.org/10.1016/j.jid.2016.04.004
Gilhar A, Landau M, Assy B, Shalaginov R, Serafimovich S, Kalish RS (2001) Melanocyte-associated T cell epitopes can function as autoantigens for transfer of alopecia areata to human scalp explants on Prkdc(scid) mice. J Invest Dermatol 117(6):1357–1362. https://doi.org/10.1046/j.0022-202x.2001.01583.x
Yenin JZ, Serarslan G, Yonden Z, Ulutas KT (2015) Investigation of oxidative stress in patients with alopecia areata and its relationship with disease severity, duration, recurrence and pattern. Clin Exp Dermatol 40(6):617–621. https://doi.org/10.1111/ced.12556
Ozturk P, Arican O, Kurutas EB, Mulayim K (2016) Oxidative stress biomarkers and adenosine deaminase over the alopecic area of the patients with alopecia areata. Balkan Med J 33(2):188–192. https://doi.org/10.5152/balkanmedj.2016.16190
Acharya P, Mathur MC (2020) Oxidative stress in alopecia areata: a systematic review and meta-analysis. Int J Dermatol 59(4):434–440. https://doi.org/10.1111/ijd.14753
Andersen YM, Egeberg A, Gislason GH, Skov L, Thyssen JP (2017) Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol 76(2):274–280 e271. https://doi.org/10.1016/j.jaad.2016.08.047
Mohan GC, Silverberg JI (2015) Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol 151(5):522–528. https://doi.org/10.1001/jamadermatol.2014.3324
Barahmani N, Schabath MB, Duvic M, National Alopecia Areata R (2009) History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol 61(4):581–591. https://doi.org/10.1016/j.jaad.2009.04.031
Zhao Y, Zhang B, Caulloo S, Chen X, Li Y, Zhang X (2012) Diffuse alopecia areata is associated with intense inflammatory infiltration and CD8+ T cells in hair loss regions and an increase in serum IgE level. Indian J Dermatol Venereol Leprol 78(6):709–714. https://doi.org/10.4103/0378-6323.102361
Li SF, Zhang XT, Qi SL, Ye YT, Cao H, Yang YQ, McElwee KJ, Zhang X (2015) Allergy to dust mites may contribute to early onset and severity of alopecia areata. Clin Exp Dermatol 40(2):171–176. https://doi.org/10.1111/ced.12471
Zhang X, McElwee KJ (2020) Allergy promotes alopecia areata in a subset of patients. Exp Dermatol 29(3):239–242. https://doi.org/10.1111/exd.14027
Lee YB, Lee WS (2020) Efficacy of antihistamines in combination with topical corticosteroid and superficial cryotherapy for treatment of alopecia areata: a retrospective cohort study. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.06.1026
Ohyama M, Shimizu A, Tanaka K, Amagai M (2010) Experimental evaluation of ebastine, a second-generation anti-histamine, as a supportive medication for alopecia areata. J Dermatol Sci 58(2):154–157. https://doi.org/10.1016/j.jdermsci.2010.03.009
Marks DH, Mesinkovska N, Senna MM (2019) Cause or cure? Review of dupilumab and alopecia areata. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2019.06.010
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535(7610):75–84. https://doi.org/10.1038/nature18848
McElwee KJ, Niiyama S, Freyschmidt-Paul P, Wenzel E, Kissling S, Sundberg JP, Hoffmann R (2003) Dietary soy oil content and soy-derived phytoestrogen genistein increase resistance to alopecia areata onset in C3H/HeJ mice. Exp Dermatol 12(1):30–36. https://doi.org/10.1034/j.1600-0625.2003.120104.x
Moreno-Arrones OM, Serrano-Villar S, Perez-Brocal V, Saceda-Corralo D, Morales-Raya C, Rodrigues-Barata R, Moya A, Jaen-Olasolo P, Vano-Galvan S (2020) Analysis of the gut microbiota in alopecia areata: identification of bacterial biomarkers. J Eur Acad Dermatol Venereol 34(2):400–405. https://doi.org/10.1111/jdv.15885
Lousada MB, Lachnit T, Edelkamp J, Rouille T, Ajdic D, Uchida Y, Di Nardo A, Bosch TCG, Paus R (2020) Exploring the human hair follicle microbiome. Br J Dermatol. https://doi.org/10.1111/bjd.19461
Pinto D, Sorbellini E, Marzani B, Rucco M, Giuliani G, Rinaldi F (2019) Scalp bacterial shift in Alopecia areata. PLoS One 14(4):e0215206. https://doi.org/10.1371/journal.pone.0215206
Wang EHC, DeStefano GM, Patel AV, Drill E, Harel S, Cela C, Tavazoie M, Christiano AM (2017) Identification of differentially expressed miRNAs in alopecia areata that target immune-regulatory pathways. Genes Immun 18(2):100–104. https://doi.org/10.1038/gene.2017.4
Hedrich CM, Tsokos GC (2011) Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol Med 17(12):714–724. https://doi.org/10.1016/j.molmed.2011.07.005
Deng Q, Luo Y, Chang C, Wu H, Ding Y, Xiao R (2019) The emerging epigenetic role of CD8+T cells in autoimmune diseases: a systematic review. Front Immunol 10:856. https://doi.org/10.3389/fimmu.2019.00856
Zhao M, Liang G, Wu X, Wang S, Zhang P, Su Y, Yin H, Tan Y, Zhang J, Lu Q (2012) Abnormal epigenetic modifications in peripheral blood mononuclear cells from patients with alopecia areata. Br J Dermatol 166(2):226–273. https://doi.org/10.1111/j.1365-2133.2011.10646.x
Tafazzoli A, Forstner AJ, Broadley D, Hofmann A, Redler S, Petukhova L, Giehl KA, Kruse R, Blaumeiser B, Bohm M, Bertolini M, Rossi A, Garcia Bartels N, Lutz G, Wolff H, Blume-Peytavi U, Soreq H, Christiano AM, Botchkareva NV, Nothen MM, Betz RC (2018) Genome-wide microRNA analysis implicates miR-30b/d in the etiology of alopecia areata. J Invest Dermatol 138(3):549–556. https://doi.org/10.1016/j.jid.2017.09.046
Sheng Y, Qi S, Hu R, Zhao J, Rui W, Miao Y, Ma J, Yang Q (2019) Identification of blood microRNA alterations in patients with severe active alopecia areata. J Cell Biochem 120(9):14421–14430. https://doi.org/10.1002/jcb.28700
Asz-Sigall D, Ortega-Springall MF, Smith-Pliego M, Rodriguez-Lobato E, Martinez-Velasco MA, Arenas R, Vincenzi C, Tosti A (2019) White hair in alopecia areata: clinical forms and proposed physiopathological mechanisms. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2018.12.047
Trüeb RM, Dias MFRG (2017) Alopecia areata: a comprehensive review of pathogenesis and management. Clin Rev Allergy Immunol 54(1):68–87. https://doi.org/10.1007/s12016-017-8620-9
Rebora A (2011) Alopecia areata incognita. J Am Acad Dermatol 65(6):1228. https://doi.org/10.1016/j.jaad.2009.05.001
Alessandrini A, Starace M, Bruni F, Brandi N, Baraldi C, Misciali C, Fanti PA, Piraccini BM (2019) Alopecia areata incognita and diffuse alopecia areata: clinical, trichoscopic, histopathological, and therapeutic features of a 5-year study. Dermatol Pract Concept 9(4):272–277. https://doi.org/10.5826/dpc.0904a05
Rebora A (1987) Alopecia areata incognita: a hypothesis. Dermatologica 174(5):214–218. https://doi.org/10.1159/000249182
Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Czuwara J, Rudnicka L (2009) Alopecia areata incognita: true or false? J Am Acad Dermatol 60(1):162–163. https://doi.org/10.1016/j.jaad.2008.07.062
Ramos-e-Silva M, Pirmez R (2013) Disorders of hair growth and the pilosebaceous unit: facts and controversies. Clin Dermatol 31(6):759–763. https://doi.org/10.1016/j.clindermatol.2013.06.003
Chelidze K, Lipner SR (2018) Nail changes in alopecia areata: an update and review. Int J Dermatol 57(7):776–783. https://doi.org/10.1111/ijd.13866
Kridin K, Renert-Yuval Y, Guttman-Yassky E, Cohen AD (2020) Alopecia areata is associated with atopic diathesis: results from a population-based study of 51,561 patients. J Allergy Clin Immunol Pract 8(4):1323–1328 e1321. https://doi.org/10.1016/j.jaip.2020.01.052
Lee S, Lee H, Lee CH, Lee WS (2019) Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol 80(2):466–477 e416. https://doi.org/10.1016/j.jaad.2018.07.013
Waskiel A, Rakowska A, Sikora M, Olszewska M, Rudnicka L (2018) Trichoscopy of alopecia areata: an update. J Dermatol 45(6):692–700. https://doi.org/10.1111/1346-8138.14283
Miteva M, Tosti A (2012) Hair and scalp dermatoscopy. J Am Acad Dermatol 67(5):1040–1048. https://doi.org/10.1016/j.jaad.2012.02.013
Mane M, Nath AK, Thappa DM (2011) Utility of dermoscopy in alopecia areata. Indian J Dermatol 56(4):407–411. https://doi.org/10.4103/0019-5154.84768
Yadav D, Khandpur S, Ramam M, Singh MK, Sharma VK (2018) Utility of horizontal sections of scalp biopsies in differentiating between androgenetic alopecia and alopecia areata. Dermatology 234(3–4):137–147. https://doi.org/10.1159/000490459
Peckham SJ, Sloan SB, Elston DM (2011) Histologic features of alopecia areata other than peribulbar lymphocytic infiltrates. J Am Acad Dermatol 65(3):615–620. https://doi.org/10.1016/j.jaad.2011.02.017
Childs JM, Sperling LC (2013) Histopathology of scarring and nonscarring hair loss. Dermatol Clin 31(1):43–56. https://doi.org/10.1016/j.det.2012.08.001
Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J (2010) Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol 62(2):177–188, quiz 189–190. https://doi.org/10.1016/j.jaad.2009.10.032
Watanabe-Okada E, Amagai M, Ohyama M (2014) Histopathological investigation of clinically non-affected perilesional scalp in alopecias detected unexpected spread of disease activities. J Dermatol 41(9):802–807. https://doi.org/10.1111/1346-8138.12591
Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M (2012) British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol 166(5):916–926. https://doi.org/10.1111/j.1365-2133.2012.10955.x
Lyakhovitsky A, Aronovich A, Gilboa S, Baum S, Barzilai A (2019) Alopecia areata: a long-term follow-up study of 104 patients. J Eur Acad Dermatol Venereol 33(8):1602–1609. https://doi.org/10.1111/jdv.15582
Tosti A, Bellavista S, Iorizzo M (2006) Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol 55(3):438–441. https://doi.org/10.1016/j.jaad.2006.05.008
Cranwell WC, Lai VW, Photiou L, Meah N, Wall D, Rathnayake D, Joseph S, Chitreddy V, Gunatheesan S, Sindhu K, Sharma P, Green J, Eisman S, Yip L, Jones L, Sinclair R (2019) Treatment of alopecia areata: an Australian expert consensus statement. Australas J Dermatol 60(2):163–170. https://doi.org/10.1111/ajd.12941
Olsen E, Hordinsky M, McDonald-Hull S, Price V, Roberts J, Shapiro J, Stenn K (1999) Alopecia areata investigational assessment guidelines. National Alopecia Areata Foundation. J Am Acad Dermatol 40(2 Pt 1):242–246. https://doi.org/10.1016/s0190-9622(99)70195-7
Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, Duvic M, King LE, Jr., McMichael AJ, Randall VA, Turner ML, Sperling L, Whiting DA, Norris D, National Alopecia Areata F (2004) Alopecia areata investigational assessment guidelines--part II. National Alopecia Areata Foundation. J Am Acad Dermatol 51(3):440–447. https://doi.org/10.1016/j.jaad.2003.09.032
Jang YH, Moon SY, Lee WJ, Lee SJ, Lee WK, Park BC, Kim H, Kim DW (2016) Alopecia areata progression index, a scoring system for evaluating overall hair loss activity in alopecia areata patients with pigmented hair: a development and reliability assessment. Dermatology 232(2):143–149. https://doi.org/10.1159/000442816
Wambier CG, King BA (2019) Rethinking the classification of alopecia areata. J Am Acad Dermatol 80(2):e45. https://doi.org/10.1016/j.jaad.2018.08.059
Wyrwich KW, Kitchen H, Knight S, Aldhouse NVJ, Macey J, Nunes FP, Dutronc Y, Mesinkovska N, Ko JM, King BA (2020) The Alopecia Areata Investigator Global Assessment scale: a measure for evaluating clinically meaningful success in clinical trials. Br J Dermatol 183(4):702–709. https://doi.org/10.1111/bjd.18883
Wyrwich KW, Kitchen H, Knight S, Aldhouse NVJ, Macey J, Nunes FP, Dutronc Y, Mesinkovska N, Ko JM, King BA (2020) Development of the Scalp Hair Assessment PRO measure for alopecia areata. Br J Dermatol 183(6):1065–1072. https://doi.org/10.1111/bjd.19024
Wyrwich KW, Kitchen H, Knight S, Aldhouse NVJ, Macey J, Nunes FP, Dutronc Y, Mesinkovska N, Ko JM, King BA (2020) Development of Clinician-Reported Outcome (ClinRO) and patient-reported outcome (PRO) measures for eyebrow, eyelash and nail assessment in alopecia areata. Am J Clin Dermatol 21(5):725–732. https://doi.org/10.1007/s40257-020-00545-9
Chu TW, AlJasser M, Alharbi A, Abahussein O, McElwee K, Shapiro J (2015) Benefit of different concentrations of intralesional triamcinolone acetonide in alopecia areata: an intrasubject pilot study. J Am Acad Dermatol 73(2):338–340. https://doi.org/10.1016/j.jaad.2015.04.049
Yee BE, Tong Y, Goldenberg A, Hata T (2020) Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol 82(4):1018–1021. https://doi.org/10.1016/j.jaad.2019.11.066
de Sousa VB, Arcanjo FP, Aguiar F, Vasconcelos J, Oliveira AF, Honorio A, Pontes J (2020) Intralesional betamethasone versus triamcinolone acetonide in the treatment of localized alopecia areata: a within-patient randomized controlled trial. J Dermatol Treat 1–3. https://doi.org/10.1080/09546634.2020.1788703
Carnahan MC, Goldstein DA (2000) Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol 11(6):478–483. https://doi.org/10.1097/00055735-200012000-00016
Rossi A, Muscianese M, Piraccini BM, Starace M, Carlesimo M, Mandel VD, Alessandrini A, Calvieri S, Caro G, D'Arino A, Federico A, Magri F, Pigliacelli F, Amendolagine G, Annunziata MC, Arisi MC, Astorino S, Babino G, Bardazzi F, Barruscotti S, Belloni Fortina A, Borghi A, Bruni F, Caccavale S, Calzavara-Pinton P, Cameli N, Cardone M, Carugno A, Coppola R, Dattola A, De Felici Del Giudice MB, Di Cesare A, Dika E, Di Nunno D, D'Ovidio R, Fabbrocini G, Feliciani C, Fulgione E, Galluzzo M, Garcovich S, Garelli V, Guerriero C, Hansel K, La Placa M, Lacarrubba F, Lora V, Marinello E, Megna M, Micali G, Misciali C, Monari P, Monfrecola G, Neri I, Offidani A, Orlando G, Papini M, Patrizi A, Piaserico S, Rivetti N, Simonetti O, Stan TR, Stingeni L, Talamonti M, Tassone F, Villa L, Vincenzi C, Fortuna MC (2019) Italian Guidelines in diagnosis and treatment of alopecia areata. G Ital Dermatol Venereol 154(6):609–623. https://doi.org/10.23736/S0392-0488.19.06458-7
Lenane P, Macarthur C, Parkin PC, Krafchik B, DeGroot J, Khambalia A, Pope E (2014) Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol 150(1):47–50. https://doi.org/10.1001/jamadermatol.2013.5764
Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C (2003) Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol 49(1):96–98. https://doi.org/10.1067/mjd.2003.423
Charuwichitratana S, Wattanakrai P, Tanrattanakorn S (2000) Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol 136(10):1276–1277. https://doi.org/10.1001/archderm.136.10.1276
Suchonwanit P, Kositkuljorn C, Mahasaksiri T, Leerunyakul K (2020) A comparison of the efficacy and tolerability of three corticosteroid treatment regimens in patients with alopecia areata. J Dermatol Treat 1–6. https://doi.org/10.1080/09546634.2020.1773384
Kar BR, Handa S, Dogra S, Kumar B (2005) Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol 52(2):287–290. https://doi.org/10.1016/j.jaad.2004.10.873
Vano-Galvan S, Hermosa-Gelbard A, Sanchez-Neila N, Miguel-Gomez L, Saceda-Corralo D, Rodrigues-Barata R, Ma DL, Jaen P (2016) Pulse corticosteroid therapy with oral dexamethasone for the treatment of adult alopecia totalis and universalis. J Am Acad Dermatol 74(5):1005–1007. https://doi.org/10.1016/j.jaad.2015.12.026
Shreberk-Hassidim R, Ramot Y, Gilula Z, Zlotogorski A (2016) A systematic review of pulse steroid therapy for alopecia areata. J Am Acad Dermatol 74(2):372–374 e371–375. https://doi.org/10.1016/j.jaad.2015.09.045
Cowley BJ, Dong J (2020) Use of oral corticosteroids in the treatment of alopecia areata. Arch Dis Child 105(1):96.91–98. https://doi.org/10.1136/archdischild-2019-317956
Suchonwanit P, Thammarucha S, Leerunyakul K (2019) Minoxidil and its use in hair disorders: a review. Drug Des Dev Ther 13:2777–2786. https://doi.org/10.2147/dddt.S214907
Freire PCB, Riera R, Martimbianco ALC, Petri V, Atallah AN (2019) Minoxidil for patchy alopecia areata: systematic review and meta-analysis. J Eur Acad Dermatol Venereol 33(9):1792–1799. https://doi.org/10.1111/jdv.15545
Sharma A, Michelle L, Juhasz M, Muller Ramos P, Atanaskova Mesinkovska N (2020) Low-dose oral minoxidil as treatment for non-scarring alopecia: a systematic review. Int J Dermatol 59(8):1013–1019. https://doi.org/10.1111/ijd.14933
Lee S, Kim BJ, Lee YB, Lee WS (2018) Hair regrowth outcomes of contact immunotherapy for patients with alopecia areata: a systematic review and meta-analysis. JAMA Dermatol 154(10):1145–1151. https://doi.org/10.1001/jamadermatol.2018.2312
Leong WMS, Mok ZR, Chandran NS (2020) Limited efficacy of diphenylcyclopropenone in the treatment of alopecia areata: experience from a Tertiary Healthcare Institution in Singapore. Dermatol Ther e14447. https://doi.org/10.1111/dth.14447
Zerbinati N, Esposito C, D’Este E, Calligaro A, Valsecchi R (2018) Topical immunotherapy of alopecia areata: a large retrospective study. Dermatol Ther (Heidelb) 8(1):101–110. https://doi.org/10.1007/s13555-018-0226-5
Gong Y, Zhao Y, Zhang X, Qi S, Li S, Ye Y, Yang J, Caulloo S, McElwee KJ, Zhang X (2020) Serum level of IL-4 predicts response to topical immunotherapy with diphenylcyclopropenone in alopecia areata. Exp Dermatol 29(3):231–238. https://doi.org/10.1111/exd.13758
Kim BJ, Lee S, Lee CH, Lee WS (2020) Home-based contact immunotherapy with diphenylcyclopropenone improves compliance with the recommended follow-up for patients with alopecia areata: a retrospective cohort study. J Am Acad Dermatol 82(5):1223–1225. https://doi.org/10.1016/j.jaad.2019.10.043
Lee S, Lee WS (2018) Home-based contact immunotherapy with diphenylcyclopropenone for alopecia areata is as effective and safe as clinic-based treatment in patients with stable disease: a retrospective study of 40 patients. J Am Acad Dermatol 78(3):599–601 e591. https://doi.org/10.1016/j.jaad.2017.09.037
Phan K, Ramachandran V, Sebaratnam DF (2019) Methotrexate for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol 80(1):120–127 e122. https://doi.org/10.1016/j.jaad.2018.06.064
Lai VWY, Chen G, Gin D, Sinclair R (2019) Cyclosporine for moderate-to-severe alopecia areata: a double-blind, randomized, placebo-controlled clinical trial of efficacy and safety. J Am Acad Dermatol 81(3):694–701. https://doi.org/10.1016/j.jaad.2019.04.053
Vano-Galvan S, Hermosa-Gelbard A, Sanchez-Neila N, Miguel-Gomez L, Saceda-Corralo D, Rodrigues-Barata R, Jaen P (2016) Treatment of recalcitrant adult alopecia areata universalis with oral azathioprine. J Am Acad Dermatol 74(5):1007–1008. https://doi.org/10.1016/j.jaad.2015.12.055
Lee S, Lee WS (2017) Management of alopecia areata: updates and algorithmic approach. J Dermatol 44(11):1199–1211. https://doi.org/10.1111/1346-8138.13933
Schwartz DM, Bonelli M, Gadina M, O’Shea JJ (2016) Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 12(1):25–36. https://doi.org/10.1038/nrrheum.2015.167
Howell MD, Kuo FI, Smith PA (2019) Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol 10. https://doi.org/10.3389/fimmu.2019.02342
O’Shea J, Plenge R (2012) JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36(4):542–550. https://doi.org/10.1016/j.immuni.2012.03.014
Damsky W, King B (2017) JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol 76(4):736–744. https://doi.org/10.1016/j.jaad.2016.12.005
Betz R, Petukhova L, Ripke S, Huang H, Menelaou A, Redler S, Becker T, Heilmann S, Yamany T, Duvic M, Hordinsky M, Norris D, Price V, Mackay-Wiggan J, de Jong A, DeStefano G, Moebus S, Böhm M, Blume-Peytavi U, Wolff H, Lutz G, Kruse R, Bian L, Amos C, Lee A, Gregersen P, Blaumeiser B, Altshuler D, Clynes R, de Bakker P, Nöthen M, Daly M, Christiano A (2015) Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun 6:5966. https://doi.org/10.1038/ncomms6966
Kennedy Crispin M, Ko J, Craiglow B, Li S, Shankar G, Urban J, Chen J, Cerise J, Jabbari A, Winge M, Marinkovich M, Christiano A, Oro A, King B (2016) Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI insight 1(15):e89776. https://doi.org/10.1172/jci.insight.89776
Jabbari A, Sansaricq F, Cerise J, Chen JC, Bitterman A, Ulerio G, Borbon J, Clynes R, Christiano AM, Mackay-Wiggan J (2018) An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol 138(7):1539–1545. https://doi.org/10.1016/j.jid.2018.01.032
Liu LY, Craiglow BG, Dai F, King BA (2017) Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol 76(1):22–28. https://doi.org/10.1016/j.jaad.2016.09.007
Craiglow BG, Liu LY, King BA (2017) Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol 76(1):29–32. https://doi.org/10.1016/j.jaad.2016.09.006
Craiglow BG, King BA (2019) Tofacitinib for the treatment of alopecia areata in preadolescent children. J Am Acad Dermatol 80(2):568–570. https://doi.org/10.1016/j.jaad.2018.08.041
Dai YX, Chen CC (2019) Tofacitinib therapy for children with severe alopecia areata. J Am Acad Dermatol 80(4):1164–1166. https://doi.org/10.1016/j.jaad.2018.12.041
Morales-Miranda AY, Bueno-Arias GM, Aguirre-Felix OG, Tovar-Franco R (2019) Tofacitinib as a treatment of alopecia areata in adolescents. Bol Med Hosp Infant Mex 76(4):182–187. https://doi.org/10.24875/BMHIM.19000005
Mackay-Wiggan J, Jabbari A, Nguyen N, Cerise J, Clark C, Ulerio G, Furniss M, Vaughan R, Christiano A, Clynes R (2016) Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI insight 1(15):e89790. https://doi.org/10.1172/jci.insight.89790
Jabbari A, Dai Z, Xing L, Cerise J, Ramot Y, Berkun Y, Sanchez G, Goldbach-Mansky R, Christiano A, Clynes R, Zlotogorski A (2015) Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine 2(4):351–355. https://doi.org/10.1016/j.ebiom.2015.02.015
Olamiju B, Friedmann A, King B (2019) Treatment of severe alopecia areata with baricitinib. JAAD case reports 5(10):892–894. https://doi.org/10.1016/j.jdcr.2019.07.005
Ismail FF, Sinclair R (2020) JAK inhibition in the treatment of alopecia areata - a promising new dawn? Expert Rev Clin Pharmacol 13(1):43–51. https://doi.org/10.1080/17512433.2020.1702878
Álvaro-Gracia J, García-Llorente J, Valderrama M, Gomez S, Montoro M (2020) Update on the safety profile of tofacitinib in rheumatoid arthritis from clinical trials to real-world studies: a narrative review. Rheumatol Ther. https://doi.org/10.1007/s40744-020-00258-9
Harel S, Higgins C, Cerise J, Dai Z, Chen J, Clynes R, Christiano A (2015) Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv 1(9):e1500973. https://doi.org/10.1126/sciadv.1500973
Phan K, Sebaratnam D (2019) JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol: JEADV 33(5):850–856. https://doi.org/10.1111/jdv.15489
Solimani F, Meier K, Ghoreschi K (2019) Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol 10:2847. https://doi.org/10.3389/fimmu.2019.02847
Bolduc C, Bissonnette R (2012) Safety and efficacy of adalimumab for the treatment of severe alopecia areata: case series of three patients. J Cutan Med Surg 16(4):257–260. https://doi.org/10.1177/120347541201600407
Strober B, Siu K, Alexis A, Kim G, Washenik K, Sinha A, Shupack J (2005) Etanercept does not effectively treat moderate to severe alopecia areata: an open-label study. J Am Acad Dermatol 52(6):1082–1084. https://doi.org/10.1016/j.jaad.2005.03.039
Strober B, Menon K, McMichael A, Hordinsky M, Krueger G, Panko J, Siu K, Lustgarten J, Ross E, Shapiro J (2009) Alefacept for severe alopecia areata: a randomized, double-blind, placebo-controlled study. Arch Dermatol 145(11):1262–1266. https://doi.org/10.1001/archdermatol.2009.264
Price VH, Hordinsky MK, Olsen EA, Roberts JL, Siegfried EC, Rafal ES, Korman NJ, Altrabulsi B, Leung HM, Garovoy MR, Caro I, Whiting DA (2008) Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol 58(3):395–402. https://doi.org/10.1016/j.jaad.2007.10.645
Suarez-Farinas M, Ungar B, Noda S, Shroff A, Mansouri Y, Fuentes-Duculan J, Czernik A, Zheng X, Estrada YD, Xu H, Peng X, Shemer A, Krueger JG, Lebwohl MG, Guttman-Yassky E (2015) Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol 136(5):1277–1287. https://doi.org/10.1016/j.jaci.2015.06.032
Guttman-Yassky E, Nia JK, Hashim PW, Mansouri Y, Alia E, Taliercio M, Desai PN, Lebwohl MG (2018) Efficacy and safety of secukinumab treatment in adults with extensive alopecia areata. Arch Dermatol Res 310(8):607–614. https://doi.org/10.1007/s00403-018-1853-5
Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP (2021) Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol 84(1):139–147. https://doi.org/10.1016/j.jaad.2020.08.051
Harada K, Irisawa R, Ito T, Uchiyama M, Tsuboi R (2020) The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: a case series of seven patients. Br J Dermatol 183(2):396–397. https://doi.org/10.1111/bjd.18976
Gruenstein D, Malik K, Levitt J (2020) Full scalp hair regrowth in a 4-year-old girl with alopecia areata and atopic dermatitis treated with dupilumab. JAAD Case Rep 6(12):1286–1287. https://doi.org/10.1016/j.jdcr.2020.10.010
Darrigade AS, Legrand A, Andreu N, Jacquemin C, Boniface K, Taieb A, Seneschal J (2018) Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br J Dermatol 179(2):534–536. https://doi.org/10.1111/bjd.16711
Stander S, Trense Y, Thaci D, Ludwig RJ (2020) Alopecia areata development in atopic dermatitis patients treated with dupilumab. J Eur Acad Dermatol Venereol 34(10):e612–e613. https://doi.org/10.1111/jdv.16493
Mitchell K, Levitt J (2018) Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep 4(2):143–144. https://doi.org/10.1016/j.jdcr.2017.11.020
Slowinska M, Kardynal A, Warszawik O, Czuwara J, Rudnicka L (2010) Alopecia areata developing paralell to improvement of psoriasis during ustekinumab therapy. J Dermatol Case Rep 4(1):15–17. https://doi.org/10.3315/jdcr.2010.1041
Tauber M, Beneton N, Reygagne P, Bachelez H, Viguier M (2013) Alopecia areata developing during ustekinumab therapy: report of two cases. Eur J Dermatol 23(6):912–913. https://doi.org/10.1684/ejd.2013.2221
Guttman-Yassky E, Ungar B, Noda S, Suprun M, Shroff A, Dutt R, Khattri S, Min M, Mansouri Y, Zheng X, Estrada YD, Singer GK, Suarez-Farinas M, Krueger JG, Lebwohl MG (2016) Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol 137(1):301–304. https://doi.org/10.1016/j.jaci.2015.11.001
Aleisa A, Lim Y, Gordon S, Her MJ, Zancanaro P, Abudu M, Deverapalli SC, Madani A, Rosmarin D (2019) Response to ustekinumab in three pediatric patients with alopecia areata. Pediatr Dermatol 36(1):e44–e45. https://doi.org/10.1111/pde.13699
Pagnanelli G, Cavani A, Canzona F, Mazzanti C (2020) Mild therapeutic response of alopecia areata during treatment of psoriasis with secukinumab. Eur J Dermatol 30(5):602–603. https://doi.org/10.1684/ejd.2020.3866
Eldirany SA, Myung P, Bunick CG (2020) Ixekizumab-induced alopecia areata. JAAD Case Rep 6(1):51–53. https://doi.org/10.1016/j.jdcr.2019.10.012
Yajima M, Akeda T, Kondo M, Habe K, Yamanaka K (2019) Alopecia diffusa while using interleukin-17 inhibitors against psoriasis vulgaris. Case Rep Dermatol 11(1):82–85. https://doi.org/10.1159/000499030
Poole R, Ballantyne A (2014) Apremilast: first global approval. Drugs 74(7):825–837. https://doi.org/10.1007/s40265-014-0218-4
Keren A, Shemer A, Ullmann Y, Paus R, Gilhar A (2015) The PDE4 inhibitor, apremilast, suppresses experimentally induced alopecia areata in human skin in vivo. J Dermatol Sci 77(1):74–76. https://doi.org/10.1016/j.jdermsci.2014.11.009
Taneja N, Gupta S (2020) Apremilast is efficacious in refractory alopecia areata. J Dermatolog Treat 31(7):727–729. https://doi.org/10.1080/09546634.2019.1616046
Sakakibara M, Shimoyama H, Nomura M, Nakashima C, Hirabayashi M, Sei Y, Kuwano Y (2019) Efficacy of the phosphodiesterase-4 inhibitor, apremilast, in a patient with severe alopecia areata. Eur J Dermatol 29(4):436–437. https://doi.org/10.1684/ejd.2019.3576
Mikhaylov D, Pavel A, Yao C, Kimmel G, Nia J, Hashim P, Vekaria AS, Taliercio M, Singer G, Karalekas R, Baum D, Mansouri Y, Lebwohl MG, Guttman-Yassky E (2019) A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res 311(1):29–36. https://doi.org/10.1007/s00403-018-1876-y
Zebda R, Paller AS (2018) Phosphodiesterase 4 inhibitors. J Am Acad Dermatol 78(3 Suppl 1):S43–S52. https://doi.org/10.1016/j.jaad.2017.11.056
Weber B, Radakovic S, Tanew A (2020) Apremilast for extensive and treatment-resistant alopecia areata: a retrospective analysis of five patients. Eur J Dermatol. https://doi.org/10.1684/ejd.2020.3749
NIH US (2020) National Library of Medicine. http://www.clinicaltrials.gov/
Maloney NJ, Zhao J, Tegtmeyer K, Lee EY, Cheng K (2020) Off-label studies on apremilast in dermatology: a review. J Dermatol Treat 31(2):131–140. https://doi.org/10.1080/09546634.2019.1589641
Liu LY, King BA (2017) Lack of efficacy of apremilast in 9 patients with severe alopecia areata. J Am Acad Dermatol 77(4):773–774. https://doi.org/10.1016/j.jaad.2017.05.034
Moreland L, Bate G, Kirkpatrick P (2006) Abatacept. Nat Rev Drug Discov 5(3):185–186. https://doi.org/10.1038/nrd1989
Mackay-Wiggan J, Sallee BN, Chun Wang EH, Sansaricq F, Nguyen N, Kim C, Chen JC, Christiano AM, Clynes R (2020) An open-label study evaluating the efficacy of abatacept in alopecia areata. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.09.091
Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, Banerjee S, Gladman DD (2017) Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 76(9):1550–1558. https://doi.org/10.1136/annrheumdis-2016-210724
Almohanna HM, Ahmed AA, Griggs JW, Tosti A (2020) Platelet-rich plasma in the treatment of alopecia areata: a review. J Investig Dermatol Symp Proc 20(1):S45–S49. https://doi.org/10.1016/j.jisp.2020.05.002
Li Z, Choi H, Choi D, Sohn K, Im M, Seo Y, Lee Y, Lee J, Lee Y (2012) Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg: Official Publication for Am Soc Dermatol Surg [et al] 38:1040–1046. https://doi.org/10.1111/j.1524-4725.2012.02394.x
Trink A, Sorbellini E, Bezzola P, Rodella L, Rezzani R, Ramot Y, Rinaldi F (2013) A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol 169(3):690–694. https://doi.org/10.1111/bjd.12397
El Taieb MA, Ibrahim H, Nada EA, Seif Al-Din M (2017) Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther 30(1). https://doi.org/10.1111/dth.12437
Albalat W, Ebrahim HM (2019) Evaluation of platelet-rich plasma vs intralesional steroid in treatment of alopecia areata. J Cosmet Dermatol. https://doi.org/10.1111/jocd.12858
Donovan J (2015) Successful treatment of corticosteroid-resistant ophiasis-type alopecia areata (AA) with platelet-rich plasma (PRP). JAAD Case Rep 1(5):305–307. https://doi.org/10.1016/j.jdcr.2015.07.004
Khademi F, Tehranchinia Z, Abdollahimajd F, Younespour S, Kazemi-Bajestani SMR, Taheri K (2019) The effect of platelet rich plasma on hair regrowth in patients with alopecia areata totalis: A clinical pilot study. Dermatol Ther 32(4):e12989. https://doi.org/10.1111/dth.12989
Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D (2011) Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365(22):2067–2077. https://doi.org/10.1056/NEJMoa1105143
Sharabi A, Tsokos M, Ding Y, Malek T, Klatzmann D, Tsokos G (2018) Regulatory T cells in the treatment of disease. Nat Rev Drug Discovery 17(11):823–844. https://doi.org/10.1038/nrd.2018.148
Le Duff F, Bouaziz JD, Fontas E, Ticchioni M, Viguier M, Dereure O, Reygagne P, Montaudie H, Lacour JP, Monestier S, Richard MA, Passeron T (2020) Low-dose IL-2 for treating moderate to severe alopecia areata: a 52-week multicenter prospective placebo-controlled study assessing its impact on T regulatory cell and NK cell populations. J Invest Dermatol. https://doi.org/10.1016/j.jid.2020.08.015
Cervantes J, Jimenez JJ, DelCanto GM, Tosti A (2018) Treatment of alopecia areata with simvastatin/ezetimibe. J Investig Dermatol Symp Proc 19(1):S25–S31. https://doi.org/10.1016/j.jisp.2017.10.013
Shin JM, Jung KE, Yim SH, Rao B, Hong D, Seo YJ, Kim CD, Lee Y (2020) Putative therapeutic mechanisms of simvastatin in the treatment of alopecia areata. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.03.102
Robins DN (2007) Case reports: alopecia universalis: hair growth following initiation of simvastatin and ezetimibe therapy. J Drugs Dermatol 6(9):946–947
Lattouf C, Jimenez J, Tosti A, Miteva M, Wikramanayake T, Kittles C, Herskovitz I, Handler M, Fabbrocini G, Schachner L (2015) Treatment of alopecia areata with simvastatin/ezetimibe. J Am Acad Dermatol 72(2):359–361. https://doi.org/10.1016/j.jaad.2014.11.006
Freitas Gouveia M, Trueb RM (2017) Unsuccessful Tteatment of alopecia areata with simvastatin/ezetimibe: experience in 12 patients. Skin Appendage Disord 3(3):156–160. https://doi.org/10.1159/000468991
Choi JW, Suh DW, Lew BL, Sim WY (2017) Simvastatin/ezetimibe therapy for recalcitrant alopecia areata: an open prospective study of 14 patients. Ann Dermatol 29(6):755–760. https://doi.org/10.5021/ad.2017.29.6.755
Loi C, Starace M, Piraccini BM (2016) Alopecia areata (AA) and treatment with simvastatin/ezetimibe: experience of 20 patients. J Am Acad Dermatol 74(5):e99–e100. https://doi.org/10.1016/j.jaad.2015.09.071
Morillo-Hernandez C, Lee J, English J (2019) Retrospective outcome analysis of 25 alopecia areata patients treated with simvastatin/ezetimibe. J Am Acad Dermatol 81(3):854–857. https://doi.org/10.1016/j.jaad.2019.04.047
Inui S, Nakajima T, Itami S (2007) Two cases of alopecia areata responsive to fexofenadine. J Dermatol 34(12):852–854. https://doi.org/10.1111/j.1346-8138.2007.00401.x
Inui S, Nakajima T, Toda N, Itami S (2009) Fexofenadine hydrochloride enhances the efficacy of contact immunotherapy for extensive alopecia areata: retrospective analysis of 121 cases. J Dermatol 36(6):323–327. https://doi.org/10.1111/j.1346-8138.2009.00647.x
Nori M, Iwata S, Munakata Y, Kobayashi H, Kobayashi S, Umezawa Y, Hosono O, Kawasaki H, Dang NH, Tanaka H, Shiohara T, Morimoto C (2003) Ebastine inhibits T cell migration, production of Th2-type cytokines and proinflammatory cytokines. Clin Exp Allergy 33(11):1544–1554. https://doi.org/10.1046/j.1365-2222.2003.01701.x
Johnstone MA (1997) Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol 124(4):544–547. https://doi.org/10.1016/s0002-9394(14)70870-0
Tauchi M, Fuchs TA, Kellenberger AJ, Woodward DF, Paus R, Lutjen-Drecoll E (2010) Characterization of an in vivo model for the study of eyelash biology and trichomegaly: mouse eyelash morphology, development, growth cycle, and anagen prolongation by bimatoprost. Br J Dermatol 162(6):1186–1197. https://doi.org/10.1111/j.1365-2133.2010.09685.x
Coronel-Perez IM, Rodriguez-Rey EM, Camacho-Martinez FM (2010) Latanoprost in the treatment of eyelash alopecia in alopecia areata universalis. J Eur Acad Dermatol Venereol 24(4):481–485. https://doi.org/10.1111/j.1468-3083.2009.03543.x
Ochoa BE, Sah D, Wang G, Stamper R, Price VH (2009) Instilled bimatoprost ophthalmic solution in patients with eyelash alopecia areata. J Am Acad Dermatol 61(3):530–532. https://doi.org/10.1016/j.jaad.2009.01.027
Roseborough I, Lee H, Chwalek J, Stamper RL, Price VH (2009) Lack of efficacy of topical latanoprost and bimatoprost ophthalmic solutions in promoting eyelash growth in patients with alopecia areata. J Am Acad Dermatol 60(4):705–706. https://doi.org/10.1016/j.jaad.2008.08.029
Zaher H, Gawdat H, Hegazy R, Hassan M (2015) Bimatoprost versus mometasone furoate in the treatment of scalp alopecia areata: a pilot study. Dermatology (Basel, Switzerland) 230(4):308–313. https://doi.org/10.1159/000371416
El-Ashmawy A, El-Maadawy I, El-Maghraby G (2018) Efficacy of topical latanoprost versus minoxidil and betamethasone valerate on the treatment of alopecia areata. J Dermatolog Treat 29(1):55–64. https://doi.org/10.1080/09546634.2017.1330527
Li A, Antaya R (2016) Successful treatment of pediatric alopecia areata of the scalp using topical bimatoprost. Pediatr Dermatol 33(5):e282-283. https://doi.org/10.1111/pde.12920
Park HS, Kim MW, Lee JS, Yoon HS, Huh CH, Kwon O, Cho S (2017) Oral tofacitinib monotherapy in Korean patients with refractory moderate-to-severe alopecia areata: a case series. J Am Acad Dermatol 77(5):978–980. https://doi.org/10.1016/j.jaad.2017.06.027
Almutairi N, Nour TM, Hussain NH (2019) Janus kinase inhibitors for the treatment of severe alopecia areata: an open-label comparative study. Dermatology 235(2):130–136. https://doi.org/10.1159/000494613
Bayart CB, DeNiro KL, Brichta L, Craiglow BG, Sidbury R (2017) Topical Janus kinase inhibitors for the treatment of pediatric alopecia areata. J Am Acad Dermatol 77(1):167–170. https://doi.org/10.1016/j.jaad.2017.03.024
Putterman E, Castelo-Soccio L (2018) Topical 2% tofacitinib for children with alopecia areata, alopecia totalis, and alopecia universalis. J Am Acad Dermatol 78(6):1207–1209 e1201. https://doi.org/10.1016/j.jaad.2018.02.031
Olamiju B, Friedmann A, King B (2019) Treatment of severe alopecia areata with baricitinib. JAAD Case Rep 5(10):892–894. https://doi.org/10.1016/j.jdcr.2019.07.005
Fleischmann R, Takeuchi T, Schiff M, Schlichting D, Xie L, Issa M, Stoykov I, Lisse J, Martinez-Osuna P, Rooney T, Zerbini CAF (2020) Efficacy and safety of long-term baricitinib with and without methotrexate for the treatment of rheumatoid arthritis: experience with baricitinib monotherapy continuation or after switching from methotrexate monotherapy or baricitinib plus methotrexate. Arthritis Care Res (Hoboken) 72(8):1112–1121. https://doi.org/10.1002/acr.24007
Concertpharma.com [Internet] (2019) Lexington MCP, Inc. Availble from https://ir.concertpharma.com/news-releases/news-release-details/concert-pharmaceuticals-presents-positive-phase-2-data-alopecia
Robinson MF, Damjanov N, Stamenkovic B, Radunovic G, Kivitz A, Cox L, Manukyan Z, Banfield C, Saunders M, Chandra D, Vincent MS, Mancuso J, Peeva E, Beebe JS (2020) Efficacy and safety of PF-06651600 (ritlecitinib), a novel JAK3/TEC inhibitor, in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 72(10):1621–1631. https://doi.org/10.1002/art.41316
Funding
This study was supported by the National Natural Science Foundation of China (No. 82073459 and No. 81773311).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, C., Li, X., Wang, C. et al. Alopecia Areata: an Update on Etiopathogenesis, Diagnosis, and Management. Clinic Rev Allerg Immunol 61, 403–423 (2021). https://doi.org/10.1007/s12016-021-08883-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-021-08883-0