Abstract
Allergic diseases are highly complex with respect to pathogenesis, inflammation, and response to treatment. Current efforts for allergic disease diagnosis have focused on clinical evidence as a binary outcome. Although outcome status based on clinical phenotypes (observable characteristics) is convenient and inexpensive to measure in large studies, it does not adequately provide insight into the complex molecular determinants of allergic disease. Individuals with similar clinical diagnoses do not necessarily have similar disease etiologies, natural histories, or responses to treatment. This heterogeneity contributes to the ineffective response to treatment leading to an annual estimated cost of $350 billion in the USA alone. There has been a recent focus to deconvolute the clinical heterogeneity of allergic diseases into specific endotypes using molecular and omics approaches. Endotypes are a means to classify patients based on the underlying pathophysiological mechanisms involving distinct functions or treatment response. The advent of high-throughput molecular omics, immunophenotyping, and bioinformatics methods including machine learning algorithms is facilitating the development of endotype-based diagnosis. As we move to the next decade, we should truly start treating clinical endotypes not clinical phenotype. This review highlights current efforts taking place to improve allergic disease endotyping via molecular omics profiling, immunophenotyping, and machine learning approaches in the context of precision diagnostics in allergic diseases.

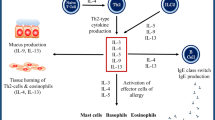
Graphical Abstract






Similar content being viewed by others
References
CDC, Gateway to Health Communication and Social Marketing Practice. Allergies. https://www.cdc.gov/healthcommunication/toolstemplates/entertainmented/tips/Allergies.html. Accessed Dec 2019.
Bacarese-Hamilton T, Gray J, Ardizzoni A, Crisanti A (2005) Allergen microarrays. Methods Mol Med 114:195–207
Pols DH, Wartna JB, van Alphen EI, Moed H, Rasenberg N, Bindels PJ, Bohnen AM (2015) Interrelationships between atopic disorders in children: a meta-analysis based on ISAAC questionnaires. PLoS One 10:e0131869
Bröms K, Norbäck D, Eriksson M, Sundelin C, Svärdsudd K (2013) Prevalence and co-occurrence of parentally reported possible asthma and allergic manifestations in pre-school children. BMC Public Health 13:764
Pinart M, Benet M, Annesi-Maesano I, von Berg A, Berdel D, Carlsen KC, Carlsen K-H, Bindslev-Jensen C, Eller E, Fantini MP (2014) Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med 2:131–140
Zheng T, Yu J, Oh MH, Zhu Z (2011) The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res 3:67–73
Hill DA, Spergel JM (2018) The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol 120:131–137
Hill DA, Grundmeier RW, Ramos M, Spergel JM (2018) Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol Pract 6:1528–1533
Lin SK, Sabharwal G, Ghaffari G (2015) A review of the evidence linking eosinophilic esophagitis and food allergy. Allergy Asthma Proc 36:26–33
Ram G, Lee J, Ott M, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, Liacouras CA, Spergel JM (2015) Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy Asthma Immunol 115(224–228):e221
Hill DA, Spergel JM (2018) Is eosinophilic esophagitis a member of the atopic march? Ann Allergy Asthma Immunol 120:113–114
Simpson A, Tan VY, Winn J, Svensén M, Bishop CM, Heckerman DE, Buchan I, Custovic A (2010) Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med 181:1200–1206
Schoos A-MM, Chawes BL, Rasmussen MA, Bloch J, Bønnelykke K, Bisgaard H (2016) Atopic endotype in childhood. J Allergy Clin Immunol 137:844–851 e844
Casale TB (2017) Biologics and biomarkers for asthma, urticaria, and nasal polyposis. J Allergy Clin Immunol 139:1411–1421
Aaron SD, Boulet LP, Reddel HK, Gershon AS (2018) Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med 198:1012–1020
Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, Sicherer S, Golden DB, Khan DA, Nicklas RA et al (2008) Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 100:S1–S148
Hamilton RG (2014) Allergic sensitization is a key risk factor for but not synonymous with allergic disease. J Allergy Clin Immunol 134:360–361
Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, Bush RK, Metcalfe DD (1988) Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol 82:986–997
Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF Jr, Wardlaw AJ, Wenzel SE, Greenberger PA (2011) Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 127:355–360
Agache I, Akdis CA (2016) Endotypes of allergic diseases and asthma: an important step in building blocks for the future of precision medicine. Allergol Int 65:243–252
Desai M, Oppenheimer J (2016) Elucidating asthma phenotypes and endotypes: progress towards personalized medicine. Ann Allergy Asthma Immunol 116:394–401
Anderson GP (2008) Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 372:1107–1119
Agache I, Akdis C, Jutel M, Virchow JC (2012) Untangling asthma phenotypes and endotypes. Allergy 67:835–846
Gupta J, Johansson E, Bernstein JA, Chakraborty R, Khurana Hershey GK, Rothenberg ME, Mersha TB (2016) Resolving the etiology of atopic disorders by using genetic analysis of racial ancestry. J Allergy Clin Immunol 138:676–699
Baye TM, Martin LJ, Hershey GKK (2010) Application of genetic/genomic approaches to allergic disorders. J Allergy Clin Immunol 126:425–436
Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R et al (2000) Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356:2144–2148
Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, Robinson D, Wenzel S, Busse W, Hansel TT et al (2007) A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 176:1062–1071
Rothenberg ME (2016) Humanized anti-IL-5 antibody therapy. Cell 165:509
Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM (2009) Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 360:985–993
Benson M (2016) Clinical implications of omics and systems medicine: focus on predictive and individualized treatment. J Intern Med 279:229–240
Shalek AK, Benson M (2017) Single-cell analyses to tailor treatments. Sci Transl Med 9
Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD (2001) Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol 108:747–752
Litonjua AA, Celedon JC, Hausmann J, Nikolov M, Sredl D, Ryan L, Platts-Mills TA, Weiss ST, Gold DR (2005) Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol 115:751–757
Celedon JC, Sredl D, Weiss ST, Pisarski M, Wakefield D, Cloutier M (2004) Ethnicity and skin test reactivity to aeroallergens among asthmatic children in Connecticut. Chest 125:85–92
Joseph CL, Ownby DR, Peterson EL, Johnson CC (2000) Racial differences in physiologic parameters related to asthma among middle-class children. Chest 117:1336–1344
Hunninghake GM, Weiss ST, Celedon JC (2006) Asthma in Hispanics. Am J Respir Crit Care Med 173:143–163
Ramsey CD, Celedon JC, Sredl DL, Weiss ST, Cloutier MM (2005) Predictors of disease severity in children with asthma in Hartford, Connecticut. Pediatr Pulmonol 39:268–275
Muraro A, Lemanske RF Jr, Castells M, Torres MJ, Khan D, Simon HU, Bindslev-Jensen C, Burks W, Poulsen LK, Sampson HA et al (2017) Precision medicine in allergic disease-food allergy, drug allergy, and anaphylaxis—PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy 72:1006–1021
Ghosh D, Bernstein JA, Khurana Hershey GK, Rothenberg ME, Mersha TB (2018) Leveraging multilayered “omics” data for atopic dermatitis: a road map to precision medicine. Front Immunol 9:2727
Libbrecht MW, Noble WS (2015) Machine learning applications in genetics and genomics. Nat Rev Genet 16:321–332
Agache I, Rogozea L (2017) Asthma biomarkers: do they bring precision medicine closer to the clinic? Allergy, Asthma Immunol Res 9:466–476
Akdis CA, Ballas ZK (2016) Precision medicine and precision health: building blocks to foster a revolutionary health care model. J Allergy Clin Immunol 137:1359–1361
Berry A, Busse WW (2016) Biomarkers in asthmatic patients: has their time come to direct treatment? J Allergy Clin Immunol 137:1317–1324
Canonica GW, Bachert C, Hellings P, Ryan D, Valovirta E, Wickman M, De Beaumont O, Bousquet J (2015) Allergen immunotherapy (AIT): a prototype of precision medicine. World Allergy Organ J 8:31
Muraro A, Lemanske RF Jr, Hellings PW, Akdis CA, Bieber T, Casale TB, Jutel M, Ong PY, Poulsen LK, Schmid-Grendelmeier P et al (2016) Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis—PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 137:1347–1358
Zissler UM, Esser-von Bieren J, Jakwerth CA, Chaker AM, Schmidt-Weber CB (2016) Current and future biomarkers in allergic asthma. Allergy 71:475–494
Saglani S (2017) Childhood severe asthma: new insights on remodelling and biomarkers. Paediatr Respir Rev 24:11–13
Richards LB, Neerincx AH, van Bragt JJ, Sterk PJ, Bel EH, Maitland-van der Zee AH (2018) Biomarkers and asthma management: analysis and potential applications. Curr Opin Allergy Clin Immunol 18:96–108
Mansouri Y, Guttman-Yassky E (2015) Immune pathways in atopic dermatitis, and definition of biomarkers through broad and targeted therapeutics. J Clin Med 4:858–873
Perlikos F, Hillas G, Loukides S (2016) Phenotyping and endotyping asthma based on biomarkers. Curr Top Med Chem 16:1582–1586
Tiotiu A (2018) Biomarkers in asthma: state of the art. Asthma Res Pract 4:10
Licari A, Castagnoli R, Brambilla I, Marseglia A, Tosca MA, Marseglia GL, Ciprandi G (2018) Asthma endotyping and biomarkers in childhood asthma. Pediatr Allergy Immunol Pulmonol 31:44–55
Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER, National Heart L, Blood Institute Severe Asthma Research, P (2010) Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 125:1028–1036 e1013
van Rensen EL, Straathof KC, Veselic-Charvat MA, Zwinderman AH, Bel EH, Sterk PJ (1999) Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax 54:403–408
Carpagnano GE, Scioscia G, Lacedonia D, Soccio P, Lepore G, Saetta M, Foschino Barbaro MP, Barnes PJ (2018) Looking for airways periostin in severe asthma: could it be useful for clustering type 2 endotype? Chest 154:1083–1090
Thijs J, Krastev T, Weidinger S, Buckens CF, de Bruin-Weller M, Bruijnzeel-Koomen C, Flohr C, Hijnen D (2015) Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol 15:453–460
Aral M, Arican O, Gul M, Sasmaz S, Kocturk SA, Kastal U, Ekerbicer HC (2006) The relationship between serum levels of total IgE, IL-18, IL-12, IFN-gamma and disease severity in children with atopic dermatitis. Mediat Inflamm 2006:73098
Trzeciak M, Glen J, Bandurski T, Sokolowska-Wojdylo M, Wilkowska A, Roszkiewicz J (2011) Relationship between serum levels of interleukin-18, IgE and disease severity in patients with atopic dermatitis. Clin Exp Dermatol 36:728–732
Morishima Y, Kawashima H, Takekuma K, Hoshika A (2010) Changes in serum lactate dehydrogenase activity in children with atopic dermatitis. Pediatr Int 52:171–174
Machura E, Rusek-Zychma M, Jachimowicz M, Wrzask M, Mazur B, Kasperska-Zajac A (2012) Serum TARC and CTACK concentrations in children with atopic dermatitis, allergic asthma, and urticaria. Pediatr Allergy Immunol 23:278–284
Johansson E, Biagini Myers JM, Martin LJ, He H, Pilipenko V, Mersha T, Weirauch M, Salomonis N, Ryan P, LeMasters GK et al (2017) KIF3A genetic variation is associated with pediatric asthma in the presence of eczema independent of allergic rhinitis. J Allergy Clin Immunol 140(595–598):e595
Marenholz I, Esparza-Gordillo J, Ruschendorf F, Bauerfeind A, Strachan DP, Spycher BD, Baurecht H, Margaritte-Jeannin P, Saaf A, Kerkhof M et al (2015) Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun 6:8804
Fornadley JA (2014) Skin testing for inhalant allergy. Int Forum Allergy Rhinol 4(Suppl 2):S41–S45
Tsybikov NN, Egorova EV, Kuznik BI, Fefelova EV, Magen E (2016) Biomarker assessment in chronic rhinitis and chronic rhinosinusitis: endothelin-1, TARC/CCL17, neopterin, and alpha-defensins. Allergy Asthma Proc 37:35–42
Sonntag HJ, Filippi S, Pipis S, Custovic A (2019) Blood biomarkers of sensitization and asthma. Front Pediatr 7:251. https://doi.org/10.3389/fped.2019.00251.
Hoffman BC, Rabinovitch N (2018) Urinary leukotriene E(4) as a biomarker of exposure, susceptibility, and risk in asthma: an update. Immunol Allergy Clin N Am 38:599–610
Carraro S, Bozzetto S, Giordano G, El Mazloum D, Stocchero M, Pirillo P, Zanconato S, Baraldi E (2018) Wheezing preschool children with early-onset asthma reveal a specific metabolomic profile. Pediatr Allergy Immunol 29:375–382
Park YH, Fitzpatrick AM, Medriano CA, Jones DP (2017) High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J Allergy Clin Immunol 139:1518–1524.e1514
Papamichael MM, Katsardis C, Erbas B, Itsiopoulos C, Tsoukalas D (2019) Urinary organic acids as biomarkers in the assessment of pulmonary function in children with asthma. Nutr Res 61:31–40
Cavaleiro Rufo J, Paciência I, Mendes FC, Farraia M, Rodolfo A, Silva D, de Oliveira Fernandes E, Delgado L, Moreira A (2019) Exhaled breath condensate volatilome allows sensitive diagnosis of persistent asthma. Allergy 74:527–534
Schleich FN, Zanella D, Stefanuto P-H, Bessonov K, Smolinska A, Dallinga JW, Henket M, Paulus V, Guissard F, Graff S et al (2019) Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am J Respir Crit Care Med 200:444–453
Menzies-Gow A, Mansur AH, Brightling CE (2020) Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J 26;55(3). https://doi.org/10.1183/13993003.01633-2019
Hamilton JD, Suárez-Fariñas M, Dhingra N, Cardinale I, Li X, Kostic A, Ming JE, Radin AR, Krueger JG, Graham N et al (2014) Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 134:1293–1300
Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Program NCS et al (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22:850–859
Tsybikov NN, Egorova EV, Kuznik BI, Fefelova EV, Magen E (2016) Biomarker assessment in chronic rhinitis and chronic rhinosinusitis: endothelin-1, TARC/CCL17, neopterin, and α-defensins. Allergy Asthma Proc 37:35–42
Badorrek P, Müller M, Koch W, Hohlfeld JM, Krug N (2017) Specificity and reproducibility of nasal biomarkers in patients with allergic rhinitis after allergen challenge chamber exposure. Ann Allergy Asthma Immunol 118:290–297
Oriel RC, Wang J (2019) Diagnosis and management of food allergy. Pediatr Clin N Am 66:941–954
Hemmings O, Kwok M, McKendry R, Santos AF (2018) Basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep 18:77–77
Bahri R, Custovic A, Korosec P, Tsoumani M, Barron M, Wu J, Sayers R, Weimann A, Ruiz-Garcia M, Patel N et al (2018) Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J Allergy Clin Immunol 142:485–496.e416
Santos AF, Couto-Francisco N, Bécares N, Kwok M, Bahnson HT, Lack G (2018) A novel human mast cell activation test for peanut allergy. J Allergy Clin Immunol 142:689–691.e689
Syed A, Garcia MA, Lyu S-C, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O'Riordan G et al (2014) Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 133:500–510
Ober C, Yao TC (2011) The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev 242:10–30
Portelli MA, Hodge E, Sayers I (2015) Genetic risk factors for the development of allergic disease identified by genome-wide association. Clin Exp Allergy 45:21–31
Bonnelykke K, Sparks R, Waage J, Milner JD (2015) Genetics of allergy and allergic sensitization: common variants, rare mutations. Curr Opin Immunol 36:115–126
Ortiz RA, Barnes KC (2015) Genetics of allergic diseases. Immunol Allergy Clin N Am 35:19–44
Andiappan AK, Sio YY, Lee B, Suri BK, Matta SA, Lum J, Foo S, Koh G, Liu J, Zolezzi F et al (2016) Functional variants of 17q12-21 are associated with allergic asthma but not allergic rhinitis. J Allergy Clin Immunol 137(758–766):e753
Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Moller E, Mercader JM, Belgrave D, den Dekker HT, Husby A, Sevelsted A, Faura-Tellez G et al (2014) A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 46:51–55
Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, Himes BE, Levin AM, Mathias RA, Hancock DB et al (2011) Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet 43:887–892
Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson W et al (2010) A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 363:1211–1221
Asai Y, Eslami A, van Ginkel CD, Akhabir L, Wan M, Yin D, Ellis G, Ben-Shoshan M, Marenholz I, Martino D et al (2018) A Canadian genome-wide association study and meta-analysis confirm HLA as a risk factor for peanut allergy independent of asthma. J Allergy Clin Immunol 141:1513–1516
Marenholz I, Grosche S, Kalb B, Ruschendorf F, Blumchen K, Schlags R, Harandi N, Price M, Hansen G, Seidenberg J et al (2017) Genome-wide association study identifies the SERPINB gene cluster as a susceptibility locus for food allergy. Nat Commun 8:1056
Liu X, Hong X, Tsai HJ, Mestan KK, Shi M, Kefi A, Hao K, Chen Q, Wang G, Caruso D et al (2018) Genome-wide association study of maternal genetic effects and parent-of-origin effects on food allergy. Medicine (Baltimore) 97:e0043
Asai Y, Eslami A, van Ginkel CD, Akhabir L, Wan M, Ellis G, Ben-Shoshan M, Martino D, Ferreira MA, Allen K et al (2018) Genome-wide association study and meta-analysis in multiple populations identifies new loci for peanut allergy and establishes C11orf30/EMSY as a genetic risk factor for food allergy. J Allergy Clin Immunol 141:991–1001
Brown SJ (2017) Molecular mechanisms in atopic eczema: insights gained from genetic studies. J Pathol 241:140–145
Osawa R, Akiyama M, Shimizu H (2011) Filaggrin gene defects and the risk of developing allergic disorders. Allergol Int 60:1–9
Brown SJ, Sandilands A, Zhao Y, Liao H, Relton CL, Meggitt SJ, Trembath RC, Barker JN, Reynolds NJ, Cordell HJ et al (2008) Prevalent and low-frequency null mutations in the filaggrin gene are associated with early-onset and persistent atopic eczema. J Invest Dermatol 128:1591–1594
Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, Mukhopadhyay S, Smith GD, Palmer CN, McLean WH et al (2008) The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol 121(872–877):e879
Kelly RS, Dahlin A, McGeachie MJ, Qiu W, Sordillo J, Wan ES, Wu AC, Lasky-Su J (2017) Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest 151:262–277
Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A4, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, Hacohen N (2017) Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356(6335). https://doi.org/10.1126/science.aah4573
Kimball AK, Oko LM, Bullock BL, Nemenoff RA, van Dyk LF, Clambey ET (2018) A beginner’s guide to analyzing and visualizing mass cytometry data. J Immunol 200:3–22
Nassar AF, Ogura H, Wisnewski AV (2015) Impact of recent innovations in the use of mass cytometry in support of drug development. Drug Discov Today 20:1169–1175
Bhattacharya S, Dunn P, Thomas CG, Smith B, Schaefer H, Chen J, Hu Z, Zalocusky KA, Shankar RD, Shen-Orr SS et al (2018) ImmPort, toward repurposing of open access immunological assay data for translational and clinical research. Sci Data 5:180015
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Bertucci F, Finetti P, Rougemont J, Charafe-Jauffret E, Cervera N, Tarpin C, Nguyen C, Xerri L, Houlgatte R, Jacquemier J et al (2005) Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res 65:2170–2178
Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P et al (2007) Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med 13:361–366
Ramaswamy S, Ross KN, Lander ES, Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33:49–54
Shivanna B, Maity S, Zhang S, Patel A, Jiang W, Wang L, Welty SE, Belmont J, Coarfa C, Moorthy B (2016) Gene expression profiling identifies cell proliferation and inflammation as the predominant pathways regulated by aryl hydrocarbon receptor in primary human fetal lung cells exposed to hyperoxia. Toxicol Sci 152:155–168
Lau C, Olstad OK, Holden M, Nygard S, Fure H, Lappegard KT, Brekke OL, Espevik T, Hovig E, Mollnes TE (2015) Gene expression profiling of gram-negative bacteria-induced inflammation in human whole blood: the role of complement and CD14-mediated innate immune response. Genomics Data 5:176–183
Hwang Y, Kim J, Shin JY, Kim JI, Seo JS, Webster MJ, Lee D, Kim S (2013) Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry 3:e321
Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM (2006) Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol 126:1071–1079
Fannin RD, Auman JT, Bruno ME, Sieber SO, Ward SM, Tucker CJ, Merrick BA, Paules RS (2005) Differential gene expression profiling in whole blood during acute systemic inflammation in lipopolysaccharide-treated rats. Physiol Genomics 21:92–104
Adarichev VA, Vermes C, Hanyecz A, Mikecz K, Bremer EG, Glant TT (2005) Gene expression profiling in murine autoimmune arthritis during the initiation and progression of joint inflammation. Arthritis Res Ther 7:R196–R207
Kan M, Shumyatcher M, Himes BE (2017) Using omics approaches to understand pulmonary diseases. Respir Res 18:149
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV (2009) T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 180:388–395
Svenningsen S, Nair P (2017) Asthma endotypes and an overview of targeted therapy for asthma. Front Med (Lausanne) 4:158
Panettieri RA Jr (2018) The role of neutrophils in asthma. Immunol Allergy Clin N Am 38:629–638
Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA (2019) Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract 8(2):549–554.e1
Institute for Clinical and Economic Review, 2018. Biologic therapies for treatment of asthma associated with type 2 inflammation: effectiveness, value, and value-based price benchmarks: final evidence report. Available from: http://resource.nlm.nih.gov/101745064. Accessed Feb 2020.
Croteau-Chonka DC, Qiu W, Martinez FD, Strunk RC, Lemanske RF Jr, Liu AH, Gilliland FD, Millstein J, Gauderman WJ, Ober C (2017) Gene expression profiling in blood provides reproducible molecular insights into asthma control. Am J Respir Crit Care Med 195:179–188
Bell E (2020) New biologic drugs for asthma: what PCPs need to know: https://www.healio.com/pediatrics/news/print/infectious-diseases-in-children. Accessed Feb 2020
Pepper AN, Renz H, Casale TB, Garn H (2017) Biologic therapy and novel molecular targets of severe asthma. J Allergy Clin Immunol Pract 5:909–916
Campo P, Rodriguez F, Sanchez-Garcia S, Barranco P, Quirce S, Perez-Frances C, Gomez-Torrijos E, Cardenas R, Olaguibel JM, Delgado J et al (2013) Phenotypes and endotypes of uncontrolled severe asthma: new treatments. J Investig Allergol Clin Immunol 23:76–88 quiz 71 p follow 88
Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID (2007) Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 62:1043–1049
Ghosh D, Ding L, Sivaprasad U, Geh E, Biagini Myers J, Bernstein JA, Khurana Hershey GK, Mersha TB (2015) Multiple transcriptome data analysis reveals biologically relevant atopic dermatitis signature genes and pathways. PLoS One 10:e0144316
Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE, Franciosi JP, Garza JM, Kaul A, King EC et al (2013) Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 145:1289–1299
Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, Collins MH, Putnam PE, Franciosi JP, von Tiehl KF et al (2013) High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol 132:378–386
Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, Hirano I, Katzka DA, Moawad FJ, Rothenberg ME et al (2016) Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 65:524–531
Shoda T, Wen T, Aceves SS, Abonia JP, Atkins D, Bonis PA, Caldwell JM, Capocelli KE, Carpenter CL, Collins MH et al (2018) Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. Lancet Gastroenterol Hepatol 3:477–488
Yang IV, Pedersen BS, Liu A, O’Connor GT, Teach SJ, Kattan M, Misiak RT, Gruchalla R, Steinbach SF, Szefler SJ et al (2015) DNA methylation and childhood asthma in the inner city. J Allergy Clin Immunol 136:69–80
ChenW,Wang T, Pino-YanesM, Forno E, Liang L, Yan Q, Hu D, Weeks DE, Baccarelli A, Acosta-Perez E, Eng C, Han YY, Boutaoui N, Laprise C, Davies GA, Hopkin JM, Moffatt MF, Cookson WOCM, Canino G, Burchard EG, Celedón JC (2017) An epigenome-wide association study of total serum IgE in Hispanic children. J Allergy Clin Immunol 140(2):571–577. https://doi.org/10.1016/j.jaci.2016.11.030
Liang L, Willis-Owen SA, Laprise C, Wong KC, Davies GA, Hudson TJ, Binia A, Hopkin JM, Yang IV, Grundberg E (2015) An epigenome-wide association study of total serum immunoglobulin E concentration. Nature 520:670–674
DeVries A, Wlasiuk G, Miller SJ, Bosco A, Stern DA, Lohman IC, Rothers J, Jones AC, Nicodemus-Johnson J, Vasquez MM et al (2017) Epigenome-wide analysis links SMAD3 methylation at birth to asthma in children of asthmatic mothers. J Allergy Clin Immunol 140:534–542
Schofield JPR, Burg D, Nicholas B, Strazzeri F, Brandsma J, Staykova D, Folisi C, Bansal AT, Xian Y, Guo Y et al (2019) Stratification of asthma phenotypes by airway proteomic signatures. J Allergy Clin Immunol 144:70–82
Weitoft M, Muller C, Ahrman E, Bjermer L, Hoffmann HJ, Erjefalt J, Westergren-Thorsson G (2019) Comparison of normal and metaplastic epithelium in patients with stable versus persistently symptomatic severe asthma using laser-capture microdissection and data-independent acquisition-mass spectrometry. Am J Pathol 189:2358–2365
Pavel AB, Zhou L, Diaz A, Ungar B, Dan J, He H, Estrada YD, Xu H, Fernandes M, Renert-Yuval Y, Krueger JG, Guttman-Yassky E (2020) The proteomic skin profile ofmoderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol 82(3):690–699. https://doi.org/10.1016/j.jaad.2019.10.039
Leonard A, Wang J, Yu L, Liu H, Estrada Y, Greenlees L, McPhee R, Ruzin A, Guttman-Yassky E, Howell MD (2019) Atopic dermatitis endotypes based on allergen sensitization, reactivity to Staphylococcus aureus antigens, and underlying systemic inflammation. J Allergy Clin Immunol Pract 8(1):236–247.e3
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535:75–84
Borbet TC, Zhang X, Muller A, Blaser MJ (2019) The role of the changing human microbiome in the asthma pandemic. J Allergy Clin Immunol 144:1457–1466
Gschwendtner S, Kang H, Thiering E, Kublik S, Fosel B, Schulz H, Krauss-Etschmann S, Heinrich J, Scholer A, Schloter M et al (2019) Early life determinants induce sustainable changes in the gut microbiome of six-year-old children. Sci Rep 9:12675
Rachid R, Chatila TA (2016) The role of the gut microbiota in food allergy. Curr Opin Pediatr 28:748–753
Kourosh A, Luna RA, Balderas M, Nance C, Anagnostou A, Devaraj S, Davis CM (2018) Fecal microbiome signatures are different in food-allergic children compared to siblings and healthy children. Pediatr Allergy Immunol 29:545–554
Powers CE, McShane DB, Gilligan PH, Burkhart CN, Morrell DS (2015) Microbiome and pediatric atopic dermatitis. J Dermatol 42:1137–1142
Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC (2012) Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol 129:434–440 440 e431-432
Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC (2014) Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy 44:842–850
Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, Jo JH, Segre JA, Kong HH, Irvine AD (2017) Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol 139:166–172
Chng KR, Tay AS, Li C, Ng AH, Wang J, Suri BK, Matta SA, McGovern N, Janela B, Wong XF et al (2016) Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol 1:16106
Cecchi L, D'Amato G, Annesi-Maesano I (2018) External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol 141:846–857
Subramanian A, Khatri SB (2019) The exposome and asthma. Clin Chest Med 40:107–123
Siroux V, Agier L, Slama R (2016) The exposome concept: a challenge and a potential driver for environmental health research. Eur Respir Rev 25:124–129
Vrijheid M (2014) The exposome: a new paradigm to study the impact of environment on health. Thorax 69:876–878
Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den Hazel P, Thomsen C, Wright J, Athersuch TJ, Avellana N et al (2014) The human early-life exposome (HELIX): project rationale and design. Environ Health Perspect 122:535–544
Vineis P, Chadeau-Hyam M, Gmuender H, Gulliver J, Herceg Z, Kleinjans J, Kogevinas M, Kyrtopoulos S, Nieuwenhuijsen M, Phillips DH et al (2017) The exposome in practice: design of the EXPOsOMICS project. Int J Hyg Environ Health 220:142–151
Anto JM, Bousquet J, Akdis M, Auffray C, Keil T, Momas I, Postma DS, Valenta R, Wickman M, Cambon-Thomsen A (2017) Mechanisms of the development of allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol 139:388–399
Karjalainen T (2014) Bridging the science-policy gap: EU-funded research for better environmental health. Available from: https://op.europa.eu/en/publication-detail/-/publication/593b6ff1-882c-4adf-899f-43a56b80a221. Accessed Mar 2020
Cui Y, Balshaw DM, Kwok RK, Thompson CL, Collman GW, Birnbaum LS (2016) The exposome: embracing the complexity for discovery in environmental health. Environ Health Perspect 124:A137–A140
Agache I, Miller R, Gern JE, Hellings PW, Jutel M, Muraro A, Phipatanakul W, Quirce S, Peden D (2019) Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a PRACTALL document. Allergy 74:449–463
Ardern-Jones MR. Characterisation of atopic dermatitis (AD) endotypes and novel treatment targets: towards a molecular classification. Exp Dermatol. 2018 Apr;27(4):433-434. https://doi.org/10.1111/exd.13152. Epub 2016 Dec 27.
Thijs JL, Strickland I, Bruijnzeel-Koomen CAFM, Nierkens S, Giovannone B, Csomor E, Sellman BR, Mustelin T, Sleeman MA, de Bruin-Weller MS, Herath A, Drylewicz J, May RD, Hijnen D (2017) Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. J Allergy Clin Immunol 140(3):730-737. https://doi.org/10.1016/j.jaci.2017.03.023
Higdon R, Earl RK, Stanberry L, Hudac CM, Montague E, Stewart E, Janko I, Choiniere J, Broomall W, Kolker N et al (2015) The promise of multi-omics and clinical data integration to identify and target personalized healthcare approaches in autism spectrum disorders. Omics 19:197–208
Mirza B, Wang W, Wang J, Choi H, Chung NC, Ping P (2019) Machine learning and integrative analysis of biomedical big data. Genes (Basel) 10(2). https://doi.org/10.3390/genes10020087
Huang SM, Abernethy DR, Wang Y, Zhao P, Zineh I (2013) The utility of modeling and simulation in drug development and regulatory review. J Pharm Sci 102:2912–2923
Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C, Compton CH, Bisgaard H, Bucchioni E, Caruso M, Chanez P, Dahlen B, Dahlen S-E, Dyson K, Frey U, Geiser T, de Verdier MG, Gibeon D, Guo Y-k, Hashimoto S, Hedlin G, Jeyasingham E, Hekking P-PW, Higenbottam T, Horvath I, Knox AJ, Krug N, Erpenbeck VJ, Larsson LX, Lazarinis N, Matthews JG, Middelveld R, Montuschi P, Musial J, Myles D, Pahus L, Sandstrom T, Seibold W, Singer F, Strandberg K, Vestbo J, Vissing N, von Garnier C, Adcock IM, Wagers S, Rowe A, Howarth P, Wagener AH, Djukanovic R, Sterk PJ, Kian Fan Chung on behalf of the U-BIOPRED Study Group (2015) Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 46:1308–1321
Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C et al (2015) Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 46:1308–1321
Altman MC, Busse WW (2017) A deep dive into asthma transcriptomics. Lessons from U-BIOPRED. Am J Respir Crit Care Med 195:1279–1280
Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, Hoda U, Rossios C, Sousa A, Wilson SJ et al (2017) A transcriptome-driven analysis of epithelial brushings and bronchial biopsies to define asthma phenotypes in U-BIOPRED. Am J Respir Crit Care Med 195:443–455
Simpson AJ, Fowler SJ, Group, U.B.S (2018) Reclassification of bronchodilator reversibility in the U-BIOPRED adult asthma cohort using z scores. Chest 153:1070–1072
Takahashi K, Pavlidis S, Ng Kee Kwong F, Hoda U, Rossios C, Sun K, Loza M, Baribaud F, Chanez P, Fowler SJ, Horvath I, Montuschi P, Singer F, Musial J, Dahlen B, Dahlen SE, Krug N, Sandstrom T, Shaw DE, Lutter R, Bakke P, Fleming LJ, Howarth PH, Caruso M, Sousa AR, Corfield J, Auffray C, De Meulder B, Lefaudeux D, Djukanovic R, Sterk PJ, Guo Y, Adcock IM, Chung KF, on behalf of the U-BIOPRED study group (2018) Sputum proteomics and airway cell transcripts of current and ex-smokers with severe asthma in U-BIOPRED: an exploratory analysis. Eur Respir J 51(5). https://doi.org/10.1183/13993003.02173-2017
Kelly RS, Chawes BL, Blighe K, Virkud YV, Croteau-Chonka DC, McGeachie MJ, Clish CB, Bullock K, Celedón JC, Weiss ST (2018) An integrative transcriptomic and metabolomic study of lung function in children with asthma. Chest 154:335–348
Lee-Sarwar K, Kelly RS, Lasky-Su J, Moody DB, Mola AR, Cheng TY, Comstock LE, Zeiger RS, O'Connor GT, Sandel MT et al (2018) Intestinal microbial-derived sphingolipids are inversely associated with childhood food allergy. J Allergy Clin Immunol 142(335–338):e339
Zhang Y, Willis-Owen SA, Spiegel S, Lloyd CM, Moffatt MF, Cookson WO (2019) The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med 199:478–488
Obeso D, Mera-Berriatua L, Rodriguez-Coira J, Rosace D, Fernandez P, Martin-Antoniano IA, Santaolalla M, Marco Martin G, Chivato T, Fernandez-Rivas M et al (2018) Multi-omics analysis points to altered platelet functions in severe food-associated respiratory allergy. Allergy 73:2137–2149
Son MJ, Yang GJ, Jo EH, Shim YH, Kang SJ, Hong JE, Kim YE, Lee JE, Chun J, Park S et al (2019) Association of atopic dermatitis with obesity via a multi-omics approach: a protocol for a case-control study. Medicine (Baltimore) 98:e16527
Ross EG, Shah NH, Dalman RL, Nead KT, Cooke JP, Leeper NJ (2016) The use of machine learning for the identification of peripheral artery disease and future mortality risk. J Vasc Surg 64:1515–1522.e1513
Lanza ST, Rhoades BL (2013) Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev Sci 14:157–168
Havstad S, Johnson CC, Kim H, Levin AM, Zoratti EM, Joseph CL, Ownby DR, Wegienka G (2014) Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol 134:722–727. e722
Roduit C, Frei R, Depner M, Karvonen AM, Renz H, Braun-Fahrländer C, Schmausser-Hechfellner E, Pekkanen J, Riedler J, Dalphin J-C (2017) Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr 171:655–662
Dharma C, Lefebvre D, Tran M, Lou W, Subbarao P, Becker A, Mandhane P, Turvey S, Sears M, investigators, C.S (2018) Patterns of allergic sensitization and atopic dermatitis from 1 to 3 years: effects on allergic diseases. Clin Exp Allergy 48:48–59
Ross MK, Yoon J, van der Schaar A, van der Schaar M (2018) Discovering pediatric asthma phenotypes on the basis of response to controller medication using machine learning. Ann Am Thoracs Soc 15:49–58
Togias A (2003) Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol 111:1171–1183
Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R Jr, Castro M, Curran-Everett D, Fitzpatrick AM et al (2010) Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med 181:315–323
Li X, Howard TD, Moore WC, Ampleford EJ, Li H, Busse WW, Calhoun WJ, Castro M, Chung KF, Erzurum SC et al (2011) Importance of hedgehog interacting protein and other lung function genes in asthma. J Allergy Clin Immunol 127:1457–1465
National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94-138
Tang HHF, Sly PD, Holt PG, Holt KE, Inouye M (2020) Systems biology and big data in asthma and allergy: recent discoveries and emerging challenges. Eur Respir J 55(1). https://doi.org/10.1183/13993003.00844-2019
Vercelli D, Martinez FD (2006) The Faustian bargain of genetic association studies: bigger might not be better, or at least it might not be good enough. J Allergy Clin Immunol 117:1303–1305
Huang QQ, Ritchie SC, Brozynska M, Inouye M (2018) Power, false discovery rate and winner’s curse in eQTL studies. Nucleic Acids Res 46:e133
McGregor MC, Krings JG, Nair P, Castro M (2019) Role of biologics in asthma. Am J Respir Crit Care Med 199:433–445
Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M (2007) Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 370:1422–1431
Luo J, Liu D, Liu CT (2016) The efficacy and safety of antiinterleukin 13, a monoclonal antibody, in adult patients with asthma: a systematic review and meta-analysis. Medicine (Baltimore) 95:e2556
Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, Vexler V, Shames RS, Daclizumab Asthma Study, G (2008) Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. Am J Respir Crit Care Med 178:1002–1008
Farzan N, Vijverberg SJ, Kabesch M, Sterk PJ, Maitland-van der Zee AH (2018) The use of pharmacogenomics, epigenomics, and transcriptomics to improve childhood asthma management: where do we stand? Pediatr Pulmonol 53:836–845
Dhondalay GK, Rael E, Acharya S, Zhang W, Sampath V, Galli SJ, Tibshirani R, Boyd SD, Maecker H, Nadeau KC et al (2018) Food allergy and omics. J Allergy Clin Immunol 141:20–29
Barbieri R, Guryev V, Brandsma CA, Suits F, Bischoff R, Horvatovich P (2016) Proteogenomics: key driver for clinical discovery and personalized medicine. Adv Exp Med Biol 926:21–47
Jameson JL, Longo DL (2015) Precision medicine—personalized, problematic, and promising. N Engl J Med 372:2229–2234
Muraro A, Fokkens WJ, Pietikainen S, Borrelli D, Agache I, Bousquet J, Costigliola V, Joos G, Lund VJ, Poulsen LK et al (2016) European symposium on precision medicine in allergy and airways diseases: report of the European Union parliament symposium (October 14, 2015). Allergy 71:583–587
Diamant Z, Vijverberg S, Alving K, Bakirtas A, Bjermer L, Custovic A, Dahlen SE, Gaga M, Gerth van Wijk R, Giacco SD, Hamelmann E, Heaney LG, Heffler E, Kalayci Ö, Kostikas K, Lutter R, Olin AC, Sergejeva S, Simpson A, Sterk P, Tufvesson E, Agache I, Seys SF (2019) Toward clinically applicable biomarkers for asthma: An EAACI position paper. Allergy 74(10):1835-1851. https://doi.org/10.1111/all.13806
Kersten ET, Koppelman GH (2017) Pharmacogenetics of asthma: toward precision medicine. Curr Opin Pulm Med 23:12–20
Laprise C, Bouzigon E (2013) The genetics of asthma and allergic diseases: pieces of the puzzle are starting to come together. Curr Opin Allergy Clin Immunol 13:461–462
Bellanti JA, Settipane RA (2018) Allergic diseases: a collection of interactive immunologic disorders at the crossroads of genetics, environment, and immunity. Allergy Asthma Proc 39:83–85
Redwood AJ, Pavlos RK, White KD, Phillips EJ (2018) HLAs: key regulators of T-cell-mediated drug hypersensitivity. HLA 91:3–16
Thijs JL, Van Der Geest BA, Van Der Schaft J, Van Den Broek MP, Van Seggelen WO, Bruijnzeel-Koomen C, Hijnen D-J, Van Schaik RH, De Bruin-Weller M (2017) Predicting therapy response to mycophenolic acid using UGT1A9 genotyping: towards personalized medicine in atopic dermatitis. J Dermatol Treat 28:242–245
Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, Galus R, Etoh T, Mihara R, Yoshida H (2017) Anti–interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med 376:826–835
Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, Nadeau K, Kaiser S, Peters T, Perez A et al (2015) Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol 135:500–507
Wain LV, Shrine N, Artigas MS, Erzurumluoglu AM, Noyvert B, Bossini-Castillo L, Obeidat M, Henry AP, Portelli MA, Hall RJ et al (2017) Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet 49:416–425
Henry AP, Probert K, Stewart CE, Thakker D, Bhaker S, Azimi S, Hall IP, Sayers I (2019) Defining a role for lung function associated gene GSTCD in cell homeostasis. Respir Res 20:172
Rackemann FM (1947) A working classification of asthma. Am J Med 3:601–606
--- (1987) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis 136(1):225–244
Brightling CE, Woltmann G, Wardlaw AJ, Pavord ID (1999) Development of irreversible airflow obstruction in a patient with eosinophilic bronchitis without asthma. Eur Respir J 14:1228–1230
Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW (1999) Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 160:1001–1008
Wenzel SE (2012) Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18:716–725
North ML, Brook JR, Lee EY, Omana V, Daniel NM, Steacy LM, Evans GJ, Diamond ML, Ellis AK (2017) The Kingston Allergy Birth Cohort: exploring parentally reported respiratory outcomes through the lens of the exposome. Ann Allergy Asthma Immunol 118:465–473
Holzinger A, Dehmer M, Jurisica I (2014) Knowledge discovery and interactive data mining in bioinformatics—state-of-the-art, future challenges and research directions. BMC Bioinformatics 15(Suppl 6):I1
Yu KH, Snyder M (2016) Omics profiling in precision oncology. Mol Cell Proteomics 15:2525–2536
Reinke SN, Gallart-Ayala H, Gomez C, Checa A, Fauland A, Naz S, Kamleh MA, Djukanovic R, Hinks TS, Wheelock CE (2017) Metabolomics analysis identifies different metabotypes of asthma severity. Eur Respir J 49
Wills-Karp M, Ewart SL (2004) Time to draw breath: asthma-susceptibility genes are identified. Nat Rev Genet 5:376–387
Zosky GR, Sly PD (2007) Animal models of asthma. Clin Exp Allergy 37:973–988
Funding
This work was supported by the National Institutes of Health (NIH) grant R01HL132344, as well as in part by NIH R37 AI045898, U19 AI070235, R01 AI057803, R01 DK107502, P30 DK078392 (Gene and Protein Expression Core), the Campaign Urging Research for Eosinophilic Disease (CURED), and the Sunshine Charitable Foundation and its supporters, Denise and David Bunning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mersha, T.B., Afanador, Y., Johansson, E. et al. Resolving Clinical Phenotypes into Endotypes in Allergy: Molecular and Omics Approaches. Clinic Rev Allerg Immunol 60, 200–219 (2021). https://doi.org/10.1007/s12016-020-08787-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-020-08787-5