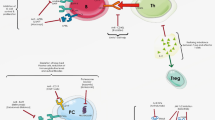
Abstract
The dysregulation of the JAK–STAT pathway is associated with various immune disorders. Four JAK inhibitors have been approved for rheumatoid arthritis (RA), and numerous JAK inhibitors are currently being tested in phase II and III trials for the treatment of various autoimmune inflammatory diseases. In this narrative review, we elucidate the involvement of the JAK–STAT signaling pathway in the pathogenesis of connective tissue diseases (CTDs). We also discuss the efficacy of the first- and second-generation JAK inhibitors (tofacitinib, baricitinib, ruxolitinib, peficitinib, filgotinib, upadacitinib, solcitinib, itacitinib, decernotinib, R333, and pf-06651600) for CTDs including RA, systemic lupus erythematosus, dermatomyositis, systemic sclerosis, Sjögren’s syndrome, and vasculitis, based on laboratory and clinical research findings. JAK inhibitors have great potential for the treatment of various CTDs by reducing multiple cytokine production and suppressing inflammation, with the advantages of rapid onset in an oral formulation and decreased corticosteroid dependence and the associated adverse events, especially in refractory cases. We also highlight the safety of novel JAK inhibitors, which can cause opportunistic infections, especially viral infections. Being a very recent therapeutic option, information regarding the safety of JAK inhibitors during pregnancy and for pediatric use is limited. However, it is recommended that JAK inhibitors should be avoided in pregnant and breastfeeding women. More clinical data, especially on highly selective inhibitors, are required to judge the efficacy and safety of JAK inhibition in CTDs.

Similar content being viewed by others
References
Mok CC (2019) The Jakinibs in systemic lupus erythematosus: progress and prospects. Expert Opin Investig Drugs 28(1):85–92. https://doi.org/10.1080/13543784.2019.1551358
Choy EH (2019) Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology 58(6):953–962. https://doi.org/10.1093/rheumatology/key339
Gadina M, Johnson C, Schwartz D, Bonelli M, Hasni S, Kanno Y, Changelian P, Laurence A, O'Shea JJ (2018) Translational and clinical advances in JAK-STAT biology: the present and future of jakinibs. J Leukoc Biol 104(3):499–514. https://doi.org/10.1002/JLB.5RI0218-084R
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ (2018) JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 17(1):78. https://doi.org/10.1038/nrd.2017.267
O'Shea JJ, Gadina M (2019) Selective Janus kinase inhibitors come of age. Nat Rev Rheumatol 15(2):74–75. https://doi.org/10.1038/s41584-018-0155-9
Baker KF, Isaacs JD (2017) Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2017-211555
Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes J, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poor G, Richez C, van Riel P, Rubbert-Roth A, Saag K, Da SJ, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D (2017) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 76(6):960–977. https://doi.org/10.1136/annrheumdis-2016-210715
Singh JA, Saag KG, Bridges SJ, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O'Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St CE, Tindall E, Miller AS, McAlindon T (2016) 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 68(1):1–25. https://doi.org/10.1002/acr.22783
Markham A (2017) Baricitinib: first global approval. Drugs 77(6):697–704. https://doi.org/10.1007/s40265-017-0723-3
Clark JD, Flanagan ME, Telliez JB (2014) Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem 57(12):5023–5038. https://doi.org/10.1021/jm401490p
Duggan S, Keam SJ (2019) Upadacitinib: first approval. Drugs. https://doi.org/10.1007/s40265-019-01211-z
Markham A, Keam SJ (2019) Peficitinib: first global approval. Drugs 79(8):887–891. https://doi.org/10.1007/s40265-019-01131-y
Ikeda K, Hayakawa K, Fujishiro M, Kawasaki M, Hirai T, Tsushima H, Miyashita T, Suzuki S, Morimoto S, Tamura N, Takamori K, Ogawa H, Sekigawa I (2017) JAK inhibitor has the amelioration effect in lupus-prone mice: the involvement of IFN signature gene downregulation. BMC Immunol 18(1). https://doi.org/10.1186/s12865-017-0225-9
Furumoto Y, Smith CK, Blanco L, Zhao W, Brooks SR, Thacker SG, Zarzour A, Sciumè G, Tsai WL, Trier AM, Nunez L, Mast L, Hoffmann V, Remaley AT, O'Shea JJ, Kaplan MJ, Gadina M (2017) Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheum 69(1):148–160. https://doi.org/10.1002/art.39818
Ripoll E, de Ramon L, Draibe BJ, Merino A, Bolanos N, Goma M, Cruzado JM, Grinyo JM, Torras J (2016) JAK3-STAT pathway blocking benefits in experimental lupus nephritis. Arthritis Res Ther 18(1):134. https://doi.org/10.1186/s13075-016-1034-x
Yamamoto M, Yokoyama Y, Shimizu Y, Yajima H, Sakurai N, Suzuki C, Naishiro Y, Takahashi H (2016) Tofacitinib can decrease anti-DNA antibody titers in inactive systemic lupus erythematosus complicated by rheumatoid arthritis. Mod Rheumatol 26(4):633–634. https://doi.org/10.3109/14397595.2015.1069473
You H, Zhang G, Wang Q, Zhang S, Zhao J, Tian X, Li H, Li M, Zeng X (2019) Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: the experience from a single centre. Ann Rheum Dis 78(10):1441–1443. https://doi.org/10.1136/annrheumdis-2019-215455
Chan ES, Herlitz LC, Jabbari A (2015) Ruxolitinib attenuates cutaneous lupus development in a mouse lupus model. J Invest Dermatol 135(7):1912–1915. https://doi.org/10.1038/jid.2015.107
de la Varga MR, Rodriguez-Bayona B, Anez GA, Medina VF, Perez VJ, Brieva JA, Rodriguez C (2017) Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur J Immunol 47(7):1211–1219. https://doi.org/10.1002/eji.201646872
Klaeschen AS, Wolf D, Brossart P, Bieber T, Wenzel J (2017) JAK inhibitor ruxolitinib inhibits the expression of cytokines characteristic of cutaneous lupus erythematosus. Exp Dermatol 26(8):728–730. https://doi.org/10.1111/exd.13253
Wenzel J, van Holt N, Maier J, Vonnahme M, Bieber T, Wolf D (2016) JAK1/2 inhibitor ruxolitinib controls a case of chilblain lupus erythematosus. J Invest Dermatol 136(6):1281–1283. https://doi.org/10.1016/j.jid.2016.02.015
Ladislau L, Suarez-Calvet X, Toquet S, Landon-Cardinal O, Amelin D, Depp M, Rodero MP, Hathazi D, Duffy D, Bondet V, Preusse C, Bienvenu B, Rozenberg F, Roos A, Benjamim CF, Gallardo E, Illa I, Mouly V, Stenzel W, Butler-Browne G, Benveniste O, Allenbach Y (2018) JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain 141(6):1609–1621. https://doi.org/10.1093/brain/awy105
Hornung T, Janzen V, Heidgen FJ, Wolf D, Bieber T, Wenzel J (2014) Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med 371(26):2537–2538. https://doi.org/10.1056/NEJMc1412997
Aeschlimann FA, Frémond M, Duffy D, Rice GI, Charuel J, Bondet V, Saire E, Neven B, Bodemer C, Balu L, Gitiaux C, Crow YJ, Bader-Meunier B (2018) A child with severe juvenile dermatomyositis treated with ruxolitinib. Brain 141(11):e80. https://doi.org/10.1093/brain/awy255
Wang K, Zhao J, Chen Z, Li T, Tan X, Zheng Y, Gu L, Guo L, Sun F, Wang H, Li J, Wang X, Riemekasten G, Ye S (2019) CD4+CXCR4+ T cells as a novel prognostic biomarker in patients with idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatology (Oxford) 58(3):557. https://doi.org/10.1093/rheumatology/key425
Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, Arima M, Maezawa R (2018) Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford) 57(12):2114–2119. https://doi.org/10.1093/rheumatology/key188
Chen Z, Wang X, Ye S (2019) Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med 381(3):291–293. https://doi.org/10.1056/NEJMc1900045
Papadopoulou C, Hong Y, Omoyinmi E, Brogan PA, Eleftheriou D (2019) Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain 142(3):e8. https://doi.org/10.1093/brain/awz005
Kurtzman DJ, Wright NA, Lin J, Femia AN, Merola JF, Patel M, Vleugels RA (2016) Tofacitinib citrate for refractory cutaneous dermatomyositis: an alternative treatment. JAMA Dermatol 152(8):944–945. https://doi.org/10.1001/jamadermatol.2016.0866
Paik JJ, Christopher-Stine L (2017) A case of refractory dermatomyositis responsive to tofacitinib. Semin Arthritis Rheum 46(4):e19. https://doi.org/10.1016/j.semarthrit.2016.08.009
Siamak Moghadam-Kia DCRA (2019) Management of refractory cutaneous dermatomyositis: potential role of Janus kinase inhibition with tofacitinib. Rheumatology. https://doi.org/10.1093/rheumatology/key366
Wendel S, Venhoff N, Frye BC, May AM, Agarwal P, Rizzi M, Voll RE, Thiel J (2019) Successful treatment of extensive calcifications and acute pulmonary involvement in dermatomyositis with the Janus-kinase inhibitor tofacitinib – a report of two cases. J Autoimmun 100:131–136. https://doi.org/10.1016/j.jaut.2019.03.003
Babaoglu H, Varan O, Atas N, Satis H, Salman R, Tufan A (2018) Tofacitinib for the treatment of refractory polymyositis. J Clin Rheumatol. https://doi.org/10.1097/RHU.0000000000000807
Komai T, Shoda H, Hanata N, Fujio K (2018) Tofacitinib rapidly ameliorated polyarthropathy in a patient with systemic sclerosis. Scand J Rheumatol 47(6):505–506. https://doi.org/10.1080/03009742.2017.1387673
Dees C, Tomcik M, Palumbo-Zerr K, Distler A, Beyer C, Lang V, Horn A, Zerr P, Zwerina J, Gelse K, Distler O, Schett G, Distler JH (2012) JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor beta in systemic sclerosis. Arthritis Rheum 64(9):3006–3015. https://doi.org/10.1002/art.34500
Lee J, Lee J, Kwok SK, Baek S, Jang SG, Hong SM, Min JW, Choi SS, Lee J, Cho ML, Park SH (2018) JAK-1 inhibition suppresses interferon-induced BAFF production in human salivary gland: potential therapeutic strategy for primary Sjogren’s syndrome. Arthritis Rheum 70(12):2057–2066. https://doi.org/10.1002/art.40589
Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ, Weyand CM (2018) Inhibition of JAK-STAT signaling suppresses pathogenic immune responses in medium and large vessel vasculitis. Circulation 137(18):1934–1948. https://doi.org/10.1161/CIRCULATIONAHA.117.030423
Rimar D, Alpert A, Starosvetsky E, Rosner I, Slobodin G, Rozenbaum M, Kaly L, Boulman N, Awisat A, Ginsberg S, Zilber K, Shen-Orr SS (2016) Tofacitinib for polyarteritis nodosa: a tailored therapy. Ann Rheum Dis 75(12):2214–2216. https://doi.org/10.1136/annrheumdis-2016-209330
Meshkov AD, Novikov PI, Zhilyaev EV, Ilevsky I, Moiseev SV (2019) Tofacitinib in steroid-dependent relapsing polychondritis. Ann Rheum Dis 78(7):e72. https://doi.org/10.1136/annrheumdis-2018-213554
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23):2205–2219. https://doi.org/10.1056/NEJMra1004965
Guimaraes PM, Scavuzzi BM, Stadtlober NP, Franchi SL, Lozovoy M, Iriyoda T, Costa NT, Reiche E, Maes M, Dichi I, Simao A (2017) Cytokines in systemic lupus erythematosus: far beyond Th1/Th2 dualism lupus: cytokine profiles. Immunol Cell Biol 95(9):824–831. https://doi.org/10.1038/icb.2017.53
Lundberg MZAI (2011) Pathogenesis, classification and treatment of inflammatory myopathies. Nat Rev Rheumatol 7(5):293–306
Raja J, Denton CP (2015) Cytokines in the immunopathology of systemic sclerosis. Semin Immunopathol 37(5):543–557. https://doi.org/10.1007/s00281-015-0511-7
Psianou K, Panagoulias I, Papanastasiou AD, de Lastic A, Rodi M, Spantidea PI, Degn SE, Georgiou P, Mouzaki A (2018) Clinical and immunological parameters of Sjögren’s syndrome. Autoimmun Rev 17(10):1053–1064. https://doi.org/10.1016/j.autrev.2018.05.005
Burja B, Kuret T, Sodin-Semrl S, Lakota K, Rotar Ž, Ješe R, Mrak-Poljšak K, Žigon P, Thallinger GG, Feichtinger J, Čučnik S, Tomšič M, Praprotnik S, Hočevar A (2018) A concise review of significantly modified serological biomarkers in giant cell arteritis, as detected by different methods. Autoimmun Rev 17(2):188–194. https://doi.org/10.1016/j.autrev.2017.11.022
Nakazawa D, Masuda S, Tomaru U, Ishizu A (2019) Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol 15(2):91–101. https://doi.org/10.1038/s41584-018-0145-y
Laurent Arnaud AMJH (2014) Pathogenesis of relapsing polychondritis: a 2013 update. Autoimmun Rev 13(2):90–95. https://doi.org/10.1016/j.autrev.2013.07.005
Lauper K, Mongin D, Iannone F, Kristianslund EK, Kvien TK, Nordstrom DC, Pavelka K, Pombo-Suarez M, Rotar Z, Santos MJ, Codreanu C, Lukina G, Gale SL, John M, Luder Y, Courvoisier DS, Gabay C (2019) Comparative effectiveness of TNF inhibitors and tocilizumab with and without conventional synthetic disease-modifying antirheumatic drugs in a pan-European observational cohort of bio-naive patients with rheumatoid arthritis. Semin Arthritis Rheum. https://doi.org/10.1016/j.semarthrit.2019.06.020
Winthrop KL (2017) The emerging safety profile of JAK inhibitors in rheumatic disease. NAT REV. Rheumatol 13(5):320. https://doi.org/10.1038/nrrheum.2017.23
Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, Wen X, Shi J, McGee R, Haley PJ, Shepard S, Rodgers JD, Yeleswaram S, Hollis G, Newton RC, Metcalf B, Friedman SM, Vaddi K (2010) Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 184(9):5298–5307. https://doi.org/10.4049/jimmunol.0902819
Keystone EC, Taylor PC, Drescher E, Schlichting DE, Beattie SD, Berclaz PY, Lee CH, Fidelus-Gort RK, Luchi ME, Rooney TP, Macias WL, Genovese MC (2015) Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis 74(2):333–340. https://doi.org/10.1136/annrheumdis-2014-206478
Tanaka Y, Takeuchi T, Tanaka S, Kawakami A, Iwasaki M, Song YW, Chen YH, Wei JC, Lee SH, Rokuda M, Izutsu H, Ushijima S, Kaneko Y, Akazawa R, Shiomi T, Yamada E (2019) Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3). Ann Rheum Dis 78(10):1320–1332. https://doi.org/10.1136/annrheumdis-2019-215163
Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y (2016) Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis 75(6):1057–1064. https://doi.org/10.1136/annrheumdis-2015-208279
Westhovens R, Taylor PC, Alten R, Pavlova D, Enriquez-Sosa F, Mazur M, Greenwald M, Van der Aa A, Vanhoutte F, Tasset C, Harrison P (2017) Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis 76(6):998–1008. https://doi.org/10.1136/annrheumdis-2016-210104
Quintas-Cardama A, Kantarjian H, Cortes J, Verstovsek S (2011) Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov 10(2):127–140. https://doi.org/10.1038/nrd3264
Genovese MC, van Vollenhoven RF, Pacheco-Tena C, Zhang Y, Kinnman N (2016) VX-509 (Decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 68(1):46–55. https://doi.org/10.1002/art.39473
Kawasaki M, Fujishiro M, Yamaguchi A, Nozawa K, Kaneko H, Takasaki Y, Takamori K, Ogawa H, Sekigawa I (2011) Possible role of the JAK/STAT pathways in the regulation of T cell-interferon related genes in systemic lupus erythematosus. Lupus 20(12):1231–1239. https://doi.org/10.1177/0961203311409963
Goropevsek A, Gorenjak M, Gradisnik S, Dai K, Holc I, Hojs R, Krajnc I, Pahor A, Avcin T (2017) Increased levels of STAT1 protein in blood CD4 T cells from systemic lupus erythematosus patients are associated with perturbed homeostasis of activated CD45RA(−)FOXP3(hi) regulatory subset and follow-up disease severity. J Interf Cytokine Res 37(6):254–268. https://doi.org/10.1089/jir.2016.0040
Kubo S, Yamaoka K, Kondo M, Yamagata K, Zhao J, Iwata S, Tanaka Y (2014) The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis 73(12):2192–2198. https://doi.org/10.1136/annrheumdis-2013-203756
Kubo S, Nakayamada S, Sakata K, Kitanaga Y, Ma X, Lee S, Ishii A, Yamagata K, Nakano K, Tanaka Y (2018) Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol 9(1510). https://doi.org/10.3389/fimmu.2018.01510
Braunstein I, Klein R, Okawa J, Werth VP (2012) The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol 166(5):971–975. https://doi.org/10.1111/j.1365-2133.2012.10825.x
Wallace DJ, Furie RA, Tanaka Y, Kalunian KC, Mosca M, Petri MA, Dorner T, Cardiel MH, Bruce IN, Gomez E, Carmack T, DeLozier AM, Janes JM, Linnik MD, de Bono S, Silk ME, Hoffman RW (2018) Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 392(10143):222–231. https://doi.org/10.1016/S0140-6736(18)31363-1
Dorner T, Furie R (2019) Novel paradigms in systemic lupus erythematosus. Lancet 393(10188):2344–2358. https://doi.org/10.1016/S0140-6736(19)30546-X
Furie R, Werth VP, Merola JF, Stevenson L, Reynolds TL, Naik H, Wang W, Christmann R, Gardet A, Pellerin A, Hamann S, Auluck P, Barbey C, Gulati P, Rabah D, Franchimont N (2019) Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest 129(3):1359–1371. https://doi.org/10.1172/JCI124466
Kahl L, Patel J, Layton M, Binks M, Hicks K, Leon G, Hachulla E, Machado D, Staumont-Salle D, Dickson M, Condreay L, Schifano L, Zamuner S, van Vollenhoven RF (2016) Safety, tolerability, efficacy and pharmacodynamics of the selective JAK1 inhibitor GSK2586184 in patients with systemic lupus erythematosus. Lupus 25(13):1420–1430. https://doi.org/10.1177/0961203316640910
J. K. Presto LGOR (2018) Computerized planimetry to assess clinical responsiveness in a phase II randomized trial of topical R333 for discoid lupus erythematosus. Br J Dermatol. https://doi.org/10.1111/bjd.16337
Kato M, Ikeda K, Kageyama T, Kasuya T, Kumagai T, Furuya H, Furuta S, Tamachi T, Suto A, Suzuki K, Nakajima H (2019) Successful treatment for refractory interstitial lung disease and pneumomediastinum with multidisciplinary therapy including tofacitinib in a patient with anti-MDA5 antibody-positive dermatomyositis. J Clin Rheumatol. https://doi.org/10.1097/RHU.0000000000000984
van Vollenhoven RF, Layton M, Kahl L, Schifano L, Hachulla E, Machado D, Staumont-Salle D, Patel J (2015) DRESS syndrome and reversible liver function abnormalities in patients with systemic lupus erythematosus treated with the highly selective JAK-1 inhibitor GSK2586184. Lupus 24(6):648–649. https://doi.org/10.1177/0961203315573347
Greenberg SA (2014) Sustained autoimmune mechanisms in dermatomyositis. J Pathol 233(3):215–216. https://doi.org/10.1002/path.4355
Moneta GM, Pires Marafon D, Marasco E, Rosina S, Verardo M, Fiorillo C, Minetti C, Bracci Laudiero L, Ravelli A, De Benedetti F, Nicolai R (2019) Muscle expression of type I and type II interferons is increased in juvenile dermatomyositis and related to clinical and histologic features. Arthritis Rheum 71(6):1011–1021. https://doi.org/10.1002/art.40800
Pinal-Fernandez I, Casal-Dominguez M, Derfoul A, Pak K, Plotz P, Miller FW, Milisenda JC, Grau-Junyent JM, Selva-O'Callaghan A, Paik J, Albayda J, Christopher-Stine L, Lloyd TE, Corse AM, Mammen AL (2019) Identification of distinctive interferon gene signatures in different types of myositis. Neurology 93(12):e1193–e1204. https://doi.org/10.1212/WNL.0000000000008128
Denton CP, Khanna D (2017) Systemic sclerosis. Lancet 390(10103):1685–1699. https://doi.org/10.1016/S0140-6736(17)30933-9
Cao XY, Zhao JL, Hou Y, Wang FD, Lu ZH (2019) Janus kinase inhibitor tofacitinib is a potential therapeutic option for refractory eosinophilic fasciitis. Clin Exp Rheumatol
Kim SR, Charos A, Damsky W, Heald P, Girardi M, King BA (2018) Treatment of generalized deep morphea and eosinophilic fasciitis with the Janus kinase inhibitor tofacitinib. JAAD Case Rep 4(5):443–445. https://doi.org/10.1016/j.jdcr.2017.12.003
Ciccia F, Rizzo A, Guggino G, Cavazza A, Alessandro R, Maugeri R, Cannizzaro A, Boiardi L, Iacopino DG, Salvarani C, Triolo G (2015) Difference in the expression of IL-9 and IL-17 correlates with different histological pattern of vascular wall injury in giant cell arteritis. Rheumatology (Oxford) 54(9):1596–1604. https://doi.org/10.1093/rheumatology/kev102
Genovese MC, Rubbert-Roth A, Smolen JS, Kremer J, Khraishi M, Gomez-Reino J, Sebba A, Pilson R, Williams S, Van Vollenhoven R (2013) Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J Rheumatol 40(6):768–780. https://doi.org/10.3899/jrheum.120687
Smolen JS, Genovese MC, Takeuchi T, Hyslop DL, Macias WL, Rooney T, Chen L, Dickson CL, Riddle CJ, Cardillo TE, Ishii T, Winthrop KL (2019) Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 46(1):7–18. https://doi.org/10.3899/jrheum.171361
Curtis JR, Xie F, Yang S, Bernatsky S, Chen L, Yun H, Winthrop K (2018) Herpes zoster in tofacitinib: risk is further increased with glucocorticoids but not methotrexate. Arthritis Care Res. https://doi.org/10.1002/acr.23769
Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z (2019) Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 78(8):1048–1054. https://doi.org/10.1136/annrheumdis-2018-214846
Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, Issa M, Salinas CA, Saifan C, Zhang X, Cardoso A, Gonzalez-Gay MA, Takeuchi T (2019) Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheum 71(7):1042–1055. https://doi.org/10.1002/art.40841
Clowse MEB, Feldman SR, Isaacs JD, Kimball AB, Strand V, Warren RB, Xibillé D, Chen Y, Frazier D, Geier J, Proulx J, Marren A (2016) Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 39(8):755–762. https://doi.org/10.1007/s40264-016-0431-z
Götestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, Da Silva J, Nelson-Piercy C, Cetin I, Costedoat-Chalumeau N, Dolhain R, Förger F, Khamashta M, Ruiz-Irastorza G, Zink A, Vencovsky J, Cutolo M, Caeyers N, Zumbühl C, Østensen M (2016) The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 75(5):795–810. https://doi.org/10.1136/annrheumdis-2015-208840
Funding
This work was supported by the Chinese National Key Research R&D Program (grant number 2017YFC0907600, 2008BAI59B02, Chinese National High Technology Research and Development Program, Ministry of Science and Technology (grant number 2012AA02A513), CAMS Innovation Fund for Medical Sciences (grant number CIFMS2019-I2M-2-008) and the Fundamental Research Funds for CAMS&PUMC (grant number 2019PT330004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
You, H., Xu, D., Zhao, J. et al. JAK Inhibitors: Prospects in Connective Tissue Diseases. Clinic Rev Allerg Immunol 59, 334–351 (2020). https://doi.org/10.1007/s12016-020-08786-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-020-08786-6