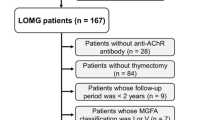
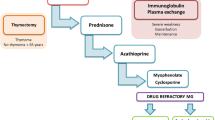
Abstract
The most common form of Myasthenia gravis (MG) is due to anti-acetylcholine receptor (AChR) antibodies and is frequently associated with thymic pathology. In this review, we discuss the immunopathological characteristics and molecular mechanisms of thymic follicular hyperplasia, the effects of corticosteroids on this thymic pathology, and the role of thymic epithelial cells (TEC), a key player in the inflammatory thymic mechanisms. This review is based not only on the literature data but also on thymic transcriptome results and analyses of pathological and immunological correlations in a vast cohort of 1035 MG patients without thymoma. We show that among patients presenting a thymic hyperplasia with germinal centers (GC), 80 % are females, indicating that thymic follicular hyperplasia is mainly a disease of women. The presence of anti-AChR antibodies is correlated with the degree of follicular hyperplasia, suggesting that the thymus is a source of anti-AChR antibodies. The degree of hyperplasia is not dependent upon the time from the onset, implying that either the antigen is chronically expressed and/or that the mechanisms of the resolution of the GC are not efficiently controlled. Glucocorticoids, a conventional therapy in MG, induce a significant reduction in the GC number, together with changes in the expression of chemokines and angiogenesis. These changes are likely related to the acetylation molecular process, overrepresented in corticosteroid-treated patients, and essential for gene regulation. Altogether, based on the pathological and molecular thymic abnormalities found in MG patients, this review provides some explanations for the benefit of thymectomy in early-onset MG patients.




Similar content being viewed by others
Abbreviations
- AChR:
-
Acetylcholine receptor
- AIRE:
-
Autoimmune regulator
- BAFF:
-
B cell-activating factor
- BCR:
-
B cell receptor
- EOMG:
-
Early-onset MG
- ER:
-
Estrogen receptor
- FDC:
-
Follicular dendritic cells
- GC:
-
Germinal center
- HEV:
-
High endothelial venules
- IFN:
-
Interferon
- IL:
-
Interleukin
- IRF:
-
IFN-regulatory factor
- KO:
-
Knock-out
- MG:
-
Myasthenia gravis
- MS:
-
Multiple sclerosis
- PBMCs:
-
Peripheral blood mononuclear cells
- PLP:
-
Proteolipid protein
- PTPN22:
-
Protein tyrosine phosphatase, non-receptor type 22
- TCR:
-
T cell receptor
- TEC:
-
Thymic epithelial cell
- Tfh:
-
T follicular helper
- TLR3:
-
Toll-like receptor 3
- TREC:
-
T cell receptor excision circles
- Treg:
-
Regulatory T cells
References
Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B (2014) Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun 48–49: 143–148.
Higuchi O, Hamuro J, Motomura M, Yamanashi Y (2011) Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 69:418–422
Zisimopoulou P, Evangelakou P, Tzartos J, Lazaridis K, Zouvelou V et al (2014) A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 52:139–145
Berrih-Aknin S (2014) Myasthenia Gravis: paradox versus paradigm in autoimmunity. J Autoimmun 52:1–28
Berrih-Aknin S, Le Panse R (2014) Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun 52:90–100
Hogquist KA, Baldwin TA, Jameson SC (2005) Central tolerance: learning self-control in the thymus. Nat Rev Immunol 5:772–782
Lopes N, Serge A, Ferrier P, Irla M (2015) Thymic Crosstalk Coordinates Medulla Organization and T-Cell Tolerance Induction. Front Immunol 6:365
Gupta S, Louis AG (2013) Tolerance and autoimmunity in primary immunodeficiency disease: a comprehensive review. Clin Rev Allergy Immunol 45:162–169
Klein L, Kyewski B, Allen PM, Hogquist KA (2014) Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 14:377–391
Laan M, Peterson P (2013) The many faces of aire in central tolerance. Front Immunol 4:326
Bertho JM, Demarquay C, Moulian N, Van Der Meeren A, Berrih-Aknin S et al (1997) Phenotypic and immunohistological analyses of the human adult thymus: evidence for an active thymus during adult life. Cell Immunol 179:30–40
Kendall MD, Johnson HR, Singh J (1980) The weight of the human thymus gland at necropsy. J Anat 131:483–497
Clarke AG, Kendall MD (1994) The thymus in pregnancy: the interplay of neural, endocrine and immune influences. Immunol Today 15:545–551
George AJ, Ritter MA (1996) Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today 17:267–272
Steinmann GG, Klaus B, Muller-Hermelink HK (1985) The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol 22:563–575
Thomas JA, Sloane JP, Imrie SF, Ritter MA, Schuurman HJ et al (1986) Immunohistology of the thymus in bone marrow transplant recipients. Am J Pathol 122:531–540
Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM et al (1998) Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690–695
Pido-Lopez J, Imami N, Aspinall R (2001) Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin Exp Immunol 125:409–413
Mitchell WA, Lang PO, Aspinall R (2010) Tracing thymic output in older individuals. Clin Exp Immunol 161:497–503
Gui J, Mustachio LM, Su DM, Craig RW (2012) Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis 3:280–290
Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP (1999) Analysis of the human thymic perivascular space during aging. J Clin Invest 104:1031–1039
Zairat’iants OV, Vetshev PS, Ippolitov I, Shkrob LO, Belokrinitskii DV et al (1991) The morphological and clinico-immunological characteristics of 2 types of myasthenia. Arkh Patol 53:22–27
Liu Z, Feng H, Yeung SC, Zheng Z, Liu W et al (2011) Extended transsternal thymectomy for the treatment of ocular myasthenia gravis. Ann Thorac Surg 92:1993–1999
Meraouna A, Cizeron-Clairac G, Panse RL, Bismuth J, Truffault F et al (2006) The chemokine CXCL13 is a key molecule in autoimmune myasthenia gravis. Blood 108:432–440
Kanda N, Tamaki K (1999) Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol 103:282–288
Medina KL, Kincade PW (1994) Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc Natl Acad Sci U S A 91:5382–5386
Mackay F, Schneider P (2009) Cracking the BAFF code. Nat Rev Immunol 9:491–502
Panchanathan R, Choubey D (2013) Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Mol Immunol 53:15–23
Straub RH (2007) The complex role of estrogens in inflammation. Endocr Rev 28:521–574
Whitacre CC, Reingold SC, O’Looney PA (1999) A gender gap in autoimmunity. Science 283:1277–1278
Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B (2002) Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest 109:1625–1633
Medina KL, Strasser A, Kincade PW (2000) Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood 95:2059–2067
Luckey D, Medina K, Taneja V (2012) B cells as effectors and regulators of sex-biased arthritis. Autoimmunity 45:364–376
Shim GJ, Kis LL, Warner M, Gustafsson JA (2004) Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci U S A 101:1720–1724
Trigunaite A, Khan A, Der E, Song A, Varikuti S et al (2013) Gr-1(high) CD11b + cells suppress B cell differentiation and lupus-like disease in lupus-prone male mice. Arthritis Rheum 65:2392–2402
Baggi F, Antozzi C, Toscani C, Cordiglieri C (2012) Acetylcholine receptor-induced experimental myasthenia gravis: what have we learned from animal models after three decades? Arch Immunol Ther Exp (Warsz) 60:19–30
Berrih S, Morel E, Gaud C, Raimond F, Le Brigand H et al (1984) Anti-AChR antibodies, thymic histology, and T cell subsets in myasthenia gravis. Neurology 34:66–71
Willcox HN, Newsom-Davis J, Calder LR (1983) Greatly increased autoantibody production in myasthenia gravis by thymocyte suspensions prepared with proteolytic enzymes. Clin Exp Immunol 54:378–386
Lisak RP, Levinson AI, Zweiman B, Kornstein MJ (1986) Antibodies to acetylcholine receptor and tetanus toxoid: in vitro synthesis by thymic lymphocytes. J Immunol 137:1221–1225
Leprince C, Cohen-Kaminsky S, Berrih-Aknin S, Vernet-Der Garabedian B, Treton D et al (1990) Thymic B cells from myasthenia gravis patients are activated B cells. Phenotypic and functional analysis. J Immunol 145:2115–2122
Fujii Y, Hashimoto J, Monden Y, Ito T, Nakahara K et al (1986) Specific activation of lymphocytes against acetylcholine receptor in the thymus in myasthenia gravis. J Immunol 136:887–891
Yoshikawa H, Satoh K, Yasukawa Y, Yamada M (2001) Analysis of immunoglobulin secretion by lymph organs with myasthenia gravis. Acta Neurol Scand 103:53–58
Schonbeck S, Padberg F, Hohlfeld R, Wekerle H (1992) Transplantation of thymic autoimmune microenvironment to severe combined immunodeficiency mice. A new model of myasthenia gravis. J Clin Invest 90:245–250
Aissaoui A, Klingel-Schmitt I, Couderc J, Chateau D, Romagne F et al (1999) Prevention of autoimmune attack by targeting specific T-cell receptors in a severe combined immunodeficiency mouse model of myasthenia gravis. Ann Neurol 46:559–567
Vincent A, Newsom-Davis J, Newton P, Beck N (1983) Acetylcholine receptor antibody and clinical response to thymectomy in myasthenia gravis. Neurology 33:1276–1282
Oosterhuis HJ, Limburg PC, Hummel-Tappel E, Van den Burg W, The TH (1985) Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 3. The effect of thymectomy. J Neurol Sci 69:335–343
Kosco-Vilbois MH, Bonnefoy JY, Chvatchko Y (1997) The physiology of murine germinal center reactions. Immunol Rev 156:127–136
Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM et al (2013) Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 38:596–605
Wakkach A, Guyon T, Bruand C, Tzartos S, Cohen-Kaminsky S et al (1996) Expression of acetylcholine receptor genes in human thymic epithelial cells: implications for myasthenia gravis. J Immunol 157:3752–3760
Wakkach A, Poea S, Chastre E, Gespach C, Lecerf F et al (1999) Establishment of a human thymic myoid cell line. Phenotypic and functional characteristics. Am J Pathol 155:1229–1240
Wittenbrink N, Klein A, Weiser AA, Schuchhardt J, Or-Guil M (2011) Is There a Typical Germinal Center? A Large-Scale Immunohistological Study on the Cellular Composition of Germinal Centers during the Hapten-Carrier-Driven Primary Immune Response in Mice. J Immunol 187:6185–6196
Shiono H, Fujii Y, Okumura M, Takeuchi Y, Inoue M et al (1997) Failure to down-regulate Bcl-2 protein in thymic germinal center B cells in myasthenia gravis. Eur J Immunol 27:805–809
Alexander CM, Tygrett LT, Boyden AW, Wolniak KL, Legge KL et al (2011) T regulatory cells participate in the control of germinal centre reactions. Immunology 133:452–468
Aloisi F, Pujol-Borrell R (2006) Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6:205–217
Armengol MP, Juan M, Lucas-Martin A, Fernandez-Figueras MT, Jaraquemada D et al (2001) Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol 159:861–873
Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P et al (2003) Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren’s syndrome. Arthritis Rheum 48:3187–3201
Randen I, Mellbye OJ, Forre O, Natvig JB (1995) The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol 41:481–486
Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14:164–174
Sugimura T, Shiokawa S, Haraoka S, Fujimoto K, Ohshima K et al (2003) Local antigen-driven oligoclonal expansion of B cells in the liver portal areas of patients with primary biliary cirrhosis. Liver Int 23:323–328
Bradshaw EM, Orihuela A, McArdel SL, Salajegheh M, Amato AA et al (2007) A local antigen-driven humoral response is present in the inflammatory myopathies. J Immunol 178:547–556
Sfikakis PP, Karali V, Lilakos K, Georgiou G, Panayiotidis P (2009) Clonal expansion of B-cells in human systemic lupus erythematosus: evidence from studies before and after therapeutic B-cell depletion. Clin Immunol 132:19–31
Berrih-Aknin S, Ragheb S, Le Panse R, Lisak RP (2013) Ectopic germinal centers, BAFF and anti-B-cell therapy in myasthenia gravis. Autoimmun Rev 12:885–893
Maecker HT, Lindstrom TM, Robinson WH, Utz PJ, Hale M et al (2012) New tools for classification and monitoring of autoimmune diseases. Nat Rev Rheumatol 8:317–328
Trevino V, Falciani F, Barrera-Saldana HA (2007) DNA microarrays: a powerful genomic tool for biomedical and clinical research. Mol Med 13:527–541
Le Panse R, Cizeron-Clairac G, Bismuth J, Berrih-Aknin S (2006) Microarrays reveal distinct gene signatures in the thymus of seropositive and seronegative myasthenia gravis patients and the role of CC chemokine ligand 21 in thymic hyperplasia. J Immunol 177:7868–7879
Cizeron-Clairac G, Le Panse R, Frenkian-Cuvelier M, Meraouna A, Truffault F et al (2008) Thymus and Myasthenia Gravis: what can we learn from DNA microarrays? J Neuroimmunol 201–202:57–63
Le Panse R, Berrih-Aknin S (2005) Thymic myoid cells protect thymocytes from apoptosis and modulate their differentiation: implication of the ERK and Akt signaling pathways. Cell Death Differ 12:463–472
Mesnard-Rouiller L, Bismuth J, Wakkach A, Poea-Guyon S, Berrih-Aknin S (2004) Thymic myoid cells express high levels of muscle genes. J Neuroimmunol 148:97–105
Nunes-Alves C, Nobrega C, Behar SM, Correia-Neves M (2013) Tolerance has its limits: how the thymus copes with infection. Trends Immunol 34:502–510
Cufi P, Dragin N, Weiss JM, Martinez-Martinez P, De Baets MH et al (2013) Implication of double-stranded RNA signaling in the etiology of autoimmune myasthenia gravis. Ann Neurol 73:281–293
Honey K, Rudensky AY (2003) Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol 3:472–482
Tolosa E, Li W, Yasuda Y, Wienhold W, Denzin LK et al (2003) Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J Clin Invest 112:517–526
Weiss JM, Cufi P, Bismuth J, Eymard B, Fadel E et al (2013) SDF-1/CXCL12 recruits B cells and antigen-presenting cells to the thymus of autoimmune myasthenia gravis patients. Immunobiology 218:373–381
Avidan N, Le Panse R, Berrih-Aknin S, Miller A (2014) Genetic basis of myasthenia gravis - a comprehensive review. J Autoimmun 52:146–153
Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP (2011) Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett 585:3689–3698
Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G et al (2011) The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest 121:3635–3644
Eschler DC, Hasham A, Tomer Y (2011) Cutting edge: the etiology of autoimmune thyroid diseases. Clin Rev Allergy Immunol 41:190–197
Lefvert AK, Zhao Y, Ramanujam R, Yu S, Pirskanen R et al (2008) PTPN22 R620W promotes production of anti-AChR autoantibodies and IL-2 in myasthenia gravis. J Neuroimmunol 197:110–113
Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT et al (2006) Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem 281:11002–11010
Hasegawa K, Martin F, Huang G, Tumas D, Diehl L et al (2004) PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 303:685–689
Rhee I, Veillette A (2012) Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol 13:439–447
Maine CJ, Marquardt K, Cheung J, La S (2014) PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J Immunol 192:1415–1424
Gradolatto A, Nazzal D, Truffault F, Bismuth J, Fadel E et al (2014) Both Treg cells and Tconv cells are defective in the Myasthenia gravis thymus: roles of IL-17 and TNF-alpha. J Autoimmun 52:53–63
Zhang X, Liu S, Chang T, Xu J, Zhang C et al (2016) Intrathymic Tfh/B Cells Interaction Leads to Ectopic GCs Formation and Anti-AChR Antibody Production: Central Role in Triggering MG Occurrence. Mol Neurobiol 53:120–131
Gilhus NE, Verschuuren JJ (2015) Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14:1023–1036
Vincent A, Palace J, Hilton-Jones D (2001) Myasthenia gravis. Lancet 357:2122–2128
Holder MJ, Knox K, Gordon J (1992) Factors modifying survival pathways of germinal center B cells. Glucocorticoids and transforming growth factor-beta, but not cyclosporin A or anti-CD19, block surface immunoglobulin-mediated rescue from apoptosis. Eur J Immunol 22:2725–2728
Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W (1992) Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology 130:3378–3386
Murray SE, Lallman HR, Heard AD, Rittenberg MB, Stenzel-Poore MP (2001) A genetic model of stress displays decreased lymphocytes and impaired antibody responses without altered susceptibility to Streptococcus pneumoniae. J Immunol 167:691–698
Murray SE, Rosenzweig HL, Johnson M, Huising MO, Sawicki K et al (2004) Overproduction of corticotropin-releasing hormone blocks germinal center formation: role of corticosterone and impaired follicular dendritic cell networks. J Neuroimmunol 156:31–41
Gomez AM, Van Den Broeck J, Vrolix K, Janssen SP, Lemmens MA et al (2010) Antibody effector mechanisms in myasthenia gravis-pathogenesis at the neuromuscular junction. Autoimmunity 43:353–370
Berrih S, Safar D, Levasseur P, Gaud C, Bach JF (1984) The in vivo effects of corticosteroids on thymocyte subsets in myasthenia gravis. J Clin Immunol 4:92–97
London J, Berrih S, Bach JF (1978) Peanut agglutinin. I. A new tool for studying T lymphocyte subpopulations. J Immunol 121:438–443
Barnes PJ (2011) Glucocorticosteroids: current and future directions. Br J Pharmacol 163:29–43
Poea-Guyon S, Christadoss P, Le Panse R, Guyon T, De Baets M et al (2005) Effects of cytokines on acetylcholine receptor expression: implications for myasthenia gravis. J Immunol 174:5941–5949
Braun D, Caramalho I, Demengeot J (2002) IFN-alpha/beta enhances BCR-dependent B cell responses. Int Immunol 14:411–419
Le Bon A, Lucas B, Vasseur F, Penit C, Papiernik M (1996) In vivo T cell response to viral superantigen. Selective migration rather than proliferation. J Immunol 156:4602–4608
Bodine SC, Furlow JD (2015) Glucocorticoids and Skeletal Muscle. Adv Exp Med Biol 872:145–176
Kalra N, Ishmael FT (2014) Cross-talk between vitamin D, estrogen and corticosteroids in glucocorticoid resistant asthma. OA inflammation 2:2–10
Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M et al (2006) Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 203:7–13
Barnes PJ, Ito K, Adcock IM (2004) Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet 363:731–733
Miller AH, Spencer RL, Pearce BD, Pisell TL, Azrieli Y et al (1998) Glucocorticoid receptors are differentially expressed in the cells and tissues of the immune system. Cell Immunol 186:45–54
Endres DB, Milholland RJ, Rosen F (1979) Sex differences in the concentrations of glucocorticoid receptors in rat liver and thymus. J Endocrinol 80:21–26
Wilder RL (1995) Neuroendocrine-immune system interactions and autoimmunity. Annu Rev Immunol 13:307–338
Fletcher AL, Lowen TE, Sakkal S, Reiseger JJ, Hammett MV et al (2009) Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. J Immunol 183:823–831
Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, et al. (2016) Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest.
Gameiro J, Nagib P, Verinaud L (2010) The thymus microenvironment in regulating thymocyte differentiation. Cell Adh Migr 4:382–390
Matsui N, Ohigashi I, Tanaka K, Sakata M, Furukawa T et al (2014) Increased number of Hassall’s corpuscles in myasthenia gravis patients with thymic hyperplasia. J Neuroimmunol 269:56–61
Aime C, Cohen-Kaminsky S, Berrih-Aknin S (1991) In vitro interleukin-1 (IL-1) production in thymic hyperplasia and thymoma from patients with myasthenia gravis. J Clin Immunol 11:268–278
Cohen-Kaminsky S, Devergne O, Delattre RM, Klingel-Schmitt I, Emilie D et al (1993) Interleukin-6 overproduction by cultured thymic epithelial cells from patients with myasthenia gravis is potentially involved in thymic hyperplasia. Eur Cytokine Netw 4:121–132
Colombara M, Antonini V, Riviera AP, Mainiero F, Strippoli R et al (2005) Constitutive activation of p38 and ERK1/2 MAPKs in epithelial cells of myasthenic thymus leads to IL-6 and RANTES overexpression: effects on survival and migration of peripheral T and B cells. J Immunol 175:7021–7028
Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP et al (2008) Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A 105:11903–11908
Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML (2010) Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood 115:5366–5375
Watanabe N, Wang YH, Lee HK, Ito T, Wang YH et al (2005) Hassall’s corpuscles instruct dendritic cells to induce CD4 + CD25+ regulatory T cells in human thymus. Nature 436:1181–1185
Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL et al (2010) Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol 184:2999–3007
Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J et al (2007) Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire + medullary thymic epithelial cells. Nat Immunol 8:351–358
Nazzal D, Gradolatto A, Truffault F, Bismuth J, Berrih-Aknin S (2014) Human thymus medullary epithelial cells promote regulatory T-cell generation by stimulating interleukin-2 production via ICOS ligand. Cell Death Dis 5:e1420
Kont V, Laan M, Kisand K, Merits A, Scott HS et al (2008) Modulation of Aire regulates the expression of tissue-restricted antigens. Mol Immunol 45:25–33
Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J et al (2004) Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med 200:1015–1026
Kurisaki H, Nagao Y, Nagafuchi S, Mitsuyama M (2013) Autoimmune gastro-pancreatitis with anti-protein disulfide isomerase-associated 2 autoantibody in Aire-deficient BALB/cAnN mice. PLoS One 8:e73862
Aharoni R, Aricha R, Eilam R, From I, Mizrahi K et al (2013) Age dependent course of EAE in Aire−/− mice. J Neuroimmunol 262:27–34
Pomie C, Vicente R, Vuddamalay Y, Lundgren BA, van der Hoek M et al (2011) Autoimmune regulator (AIRE)-deficient CD8 + CD28low regulatory T lymphocytes fail to control experimental colitis. Proc Natl Acad Sci U S A 108:12437–12442
Aricha R, Feferman T, Scott HS, Souroujon MC, Berrih-Aknin S et al (2011) The susceptibility of Aire(−/−) mice to experimental myasthenia gravis involves alterations in regulatory T cells. J Autoimmun 36:16–24
Liu Y, Zhang H, Zhang P, Meng F, Chen Y et al (2014) Autoimmune regulator expression in thymomas with or without autoimmune disease. Immunol Lett 161:50–56
Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D (2004) Cancers and immune related diseases associated with Down’s syndrome: a record linkage study. Arch Dis Child 89:1014–1017
Lima FA, Moreira-Filho CA, Ramos PL, Brentani H, Lima Lde A et al (2011) Decreased AIRE expression and global thymic hypofunction in Down syndrome. J Immunol 187:3422–3430
Greer JM, Csurhes PA, Pender MP, McCombe PA (2004) Effect of gender on T-cell proliferative responses to myelin proteolipid protein antigens in patients with multiple sclerosis and controls. J Autoimmun 22:345–352
Mantegazza R, Baggi F, Bernasconi P, Antozzi C, Confalonieri P et al (2003) Video-assisted thoracoscopic extended thymectomy and extended transsternal thymectomy (T-3b) in non-thymomatous myasthenia gravis patients: remission after 6 years of follow-up. J Neurol Sci 212:31–36
Diaz A, Black E, Dunning J (2014) Is thymectomy in non-thymomatous myasthenia gravis of any benefit? Interact Cardiovasc Thorac Surg 18:381–389
Masaoka A, Yamakawa Y, Niwa H, Fukai I, Kondo S et al (1996) Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg 62:853–859
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the FIGHT-MG (Heath-2009-242-210) grant from the European Community and a grant from the “Association Francaise contre les Myopathies” obtained by Dr S. Berrih-Aknin.
Rights and permissions
About this article
Cite this article
Truffault, F., de Montpreville, V., Eymard, B. et al. Thymic Germinal Centers and Corticosteroids in Myasthenia Gravis: an Immunopathological Study in 1035 Cases and a Critical Review. Clinic Rev Allerg Immunol 52, 108–124 (2017). https://doi.org/10.1007/s12016-016-8558-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-016-8558-3