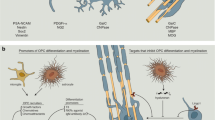
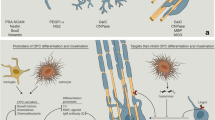
Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system that affects millions of patients worldwide. The current disease-modifying therapies (DMTs) that are widely used to treat MS only show modest effects. Because MS is a chronic disease, it is important to develop treatments that have better long-term efficacy. Recently, several new-generation DMTs have been developed, most of which target specific immune molecules based on the assumption that MS is an autoimmune disease. These DMTs are designed to inhibit inflammation that is thought to directly cause demyelination. Preliminary studies suggest that these new therapies are likely to show a greater effect in reducing relapses in early MS patients, although their long-term efficacy is still unknown. In contrast, it was recently reported that the initial course of MS does not significantly influence long-term disability and that disability increases approximately at the same rate despite variable relapse frequencies. Furthermore, new neuropathological evidence now argues against the autoimmune hypothesis and suggests that MS is a primary oligodendrogliopathy disease in which the inflammatory response may be a mere epiphenomenon. So can we be optimistic about the unproven long-term outcomes of new DMTs or should we reconsider the pathogenesis of MS when developing more disease-specific treatments?



Similar content being viewed by others
References
The IFNβ Multiple Sclerosis Study Group (1993) Interferon β-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43:655–661
The Multiple Sclerosis Collaborative Research Group (1996) Intramuscular interferon β-1a for disease progression in relapsing multiple sclerosis. Ann Neurol 39:285–294
The Copolymer 1 Multiple Sclerosis Study Group (1995) Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 45:1268–1276
Confavreux C, Vukusic S (2006) Natural history of multiple sclerosis: a unifying concept. Brain 129:606–616
Barnett MH, Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55:458–468
Henderson APD, Barnett MH, Parratt JDE et al (2009) Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 66:739–753
Furtado GC, Marcondes MC, Latkowski JA et al (2008) Swift entry of myelin-specific T lymphocytes into the central nervous system in spontaneous autoimmune encephalomyelitis. J Immunol 181:4648–4655
Cua DJ, Sherlock J, Chen Y et al (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748
Tzartos JS, Friese MA, Craner MJ et al (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells associated with active disease in multiple sclerosis. Am J Pathol 172:146–155
Reboldi A, Coisne C, Baumjohann D et al (2009) C–C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10:514–523
Esplugues E, Huber S, Gagliani N et al (2011) Controls of Th17 cells occurs in the small intestine. Nature 475:514–518
Brown DA, Sawchenko PE (2007) Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol 502:236–260
Bartholomäus I, Kawakami N, Odoardi F et al (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462:94–98
Pang Y, Campbell L, Zheng B et al (2010) Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience 166:464–475
Lieberman AP, Pitha PM, Shin HS et al (1989) Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA 86:6348–6352
Eugster HP, Frei K, Bachmann R et al (1999) Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur J Immunol 29:626–632
Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JPY (2001) TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4:1116–1122
Sriram S, Steiner I (2005) Experimental allergic encephalomyelitis: a misleading model of multiple sclerosis. Ann Neurol 58:939–945
Rivers TM, Sprunt DH, Berry GP (1933) Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. J Exp Med 58:39–53
Steinman L, Zamvil SS (2006) How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol 60:12–21
Waldor MK, Sriram S, Hardy R et al (1985) Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science 227:415–417
van Oosten BW, Lai M, Hodgkinson S et al (1997) Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology 49:351–357
Ruddle NH, Bergman CM, McGrath KM et al (1990) An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med 172:1193–1200
Selmaj K, Raine CS, Cross AH (1991) Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol 30:694–700
The Lenercept Multiple Sclerosis Study Group, The University of British Columbia MS/MRI Analysis Group (1999) TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53:457–465
Sicotte NL, Voskuhl RR (2001) Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 57:1885–1888
Robinson WH, Genovese MC, Moreland LW (2001) Demyelinating and neurologic events reported in association with tumor necrosis factor a antagonism: by what mechanisms could tumor necrosis factor a antagonists improve rheumatoid arthritis but exacerbate multiple sclerosis? Arthritis Rheum 44:1977–1983
Segal BM, Constantinescu CS, Raychaudhuri A et al (2008) Repeated subcutaneous injections of IL12/23 p40 neutralizing antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomized, dose-ranging study. Lancet Neurol 7:796–804
Lucchinetti C, Brück W, Parisi J et al (2000) Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Henderson AP, Barnett MH, Parratt JD, Prineas JW (2009) Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 66:739–753
Kotter MR, Li WW, Zhao C et al (2006) Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci 26:328–332
Rodriguez M, Warrington AE, Pease LR (2009) Human natural autoantibodies in the treatment of neurologic disease. Neurology 72:1269–1276
Nielsen HH, Toft-Hansen H, Lambertsen KL, Owens T, Finsen B (2011) Stimulation of adult oligodendrogenesis by myelin-specific T cells. Am J Pathol, doi:10.1016/j.ajpath.2011.06.006
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119:37–53
Nakahara J, Kanekura K, Nawa M et al (2009) Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J Clin Invest 119:169–181
Charcot J (1868) Histologie de la sclérose en plaque. Gazette des Hôpitaux 41:554–566
Schumacher GA, Beebe G, Kibler RF et al (1965) Problems of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci 122:552–568
Poser CM, Paty DW, Scheinberg L et al (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Vukusic S, Confavreux C (2003) Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis. J Neurol Sci 206:135–137
Confavreux C, Vukusic S (2006) Age at disability milestones in multiple sclerosis. Brain 129:595–605
Hurwitz BJ (2011) Analysis of current multiple sclerosis registries. Neurology 76:S7–S13
Brønnum-Hansen H, Koch-Henriksen N, Stenager E (2004) Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 127:844–850
Filippi M, Paty DW, Kappos L et al (1995) Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology 45:255–260
Losseff NA, Webb SL, O’Riordan JI et al (1996) Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 119:2009–2019
Fisniku LK, Chard DT, Jackson JS et al (2008) Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol 64:247–254
Fisher E, Lee JC, Nakamura K, Rudick RA (2008) Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol 64:255–265
Bonati U, Fisniku LK, Altmann DR et al (2011) Cervical cord and brain grey matter atrophy independently associate with long term MS disability. J Neurol Neurosurg Psychiatry 82:471–472
Calabrese M, Rocca MA, Atzori M et al (2010) A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol 67:376–383
Calabrese M, Filippi M, Gallo P (2010) Cortical lesion in multiple sclerosis. Nat Rev Neurol 6:438–444
Kutzelnigg A, Lucchinetti CF, Stadelmann C et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Peterson JW, Bø L, Mörk S et al (2001) Transected neuritis, apoptotic neurons and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Bø L, Vedeler CA, Nyland H, Trapp BD, Mörk S (2003) Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 4:323–331
van Horssen J, Brink BP, De Vries HE, van der Valk P, Bø L (2007) The blood-brain barrier in cortical multiple sclerosis lesions. J Neuropathol Exp Neurol 66:321–328
Magliozzi R, Howell O, Vora A et al (2007) Meningeal B-cell follicles in secondary-progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130:1089–1104
Kooi EJ, Geurts JJ, van Horssen J, Bø L, van der Valk P (2009) Meningeal inflammation is not associated with cortical demyelination in chronic multiple sclerosis. J Neuropathol Exp Neurol 68:1021–1028
Dal Biancco A, Bradl M, Frischer J et al (2008) Multiple sclerosis and Alzheimer’s disease. Ann Neurol 63:174–183
Magliozzi R, Howell OW, Reeves C et al (2010) A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68:477–493
Howell OW, Reeves CA, Nicholas R et al (2011) Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. doi:10.1093/brain/awr182, advance access
Coles AJ, Compston DA, Selmaj KW et al (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359:1786–1801
Naismith RT, Piccio L, Lyons JA et al (2010) Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology 74:1860–1867
Kappos L, Radue EW, O’Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Giovannoni G, Comi G, Cook S et al (2010) A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 362:416–426
O’Connor PW, Li D, Freedman MS et al (2006) A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66:894–900
O’Connor PW, Goodman A, Willmer-Hulme AJ et al (2004) Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology 62:2038–2043
Wynn D, Kaufmann M, Montalban X et al (2010) Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomized, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol 9:381–390
Comi G, Pulizzi A, Rovaris M et al (2008) Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 371:2085–2092
Acknowledgments
Jin Nakahara is supported by the Keio University KANRINMARU Project. This work was supported by research grant #09-24 from the National Institute of Biomedical Innovation of Japan, by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and by Grants-in-Aid for Scientific Research from MEXT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakahara, J., Maeda, M., Aiso, S. et al. Current Concepts in Multiple Sclerosis: Autoimmunity Versus Oligodendrogliopathy. Clinic Rev Allerg Immunol 42, 26–34 (2012). https://doi.org/10.1007/s12016-011-8287-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-011-8287-6