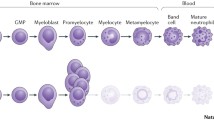
Abstract
Congenital neutropenia syndromes comprise a heterogeneous group of disorders leading to increased susceptibility to bacterial infections. Recent work has elucidated the molecular basis of several congenital neutropenia syndromes such as mutations in ELA2, HAX1, GF11, and WAS. In addition, a number of complex clinical syndromes associating congenital neutropenia have been recognized and elucidated on a genetic level, e.g. p14-deficiency or G6PC3-deficiency. The clinical and genetic findings of various neutropenia syndromes are being discussed.
Similar content being viewed by others
References
Kostmann R (1950) Hereditär reticulos-en ny systemsjukdom. Svenska Laekartidningen 47:2861–2868
Boztug K et al (2008) Congenital neutropenia syndromes. Immunol Allergy Clin North Am 28(2):259–275 vii-viii
Horwitz M et al (1999) Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic haematopoiesis. Nat Genet 23(4):433–436
Horwitz MS et al (2007) Neutrophil elastase in cyclic and severe congenital neutropenia. Blood 109(5):1817–1824
Boxer LA et al (2006) Strong evidence for autosomal dominant inheritance of severe congenital neutropenia associated with ELA2 mutations. J Pediatr 148(5):633–636
Ancliff PJ, Gale RE, Linch DC (2003) Neutrophil elastase mutations in congenital neutropenia. Hematology 8(3):165–171
Germeshausen M et al (2001) Mutations in the gene encoding neutrophil elastase (ELA2) are not sufficient to cause the phenotype of congenital neutropenia. Br J Haematol 115(1):222–224
Bellanne-Chantelot C et al (2004) Mutations in the ELA2 gene correlate with more severe expression of neutropenia: a study of 81 patients from the French Neutropenia Register. Blood 103(11):4119–4125
Skokowa J et al (2007) Severe congenital neutropenia: inheritance and pathophysiology. Curr Opin Hematol 14(1):22–28
Pham CT (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6(7):541–550
Benson KF et al (2003) Mutations associated with neutropenia in dogs and humans disrupt intracellular transport of neutrophil elastase. Nat Genet 35(1):90–96
Kollner I et al (2006) Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood 108(2):493–500
Devriendt K et al (2001) Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet 27(3):313–317
Thrasher AJ (2002) WASp in immune-system organization and function. Nat Rev Immunol 2(9):635–646
Moulding DA et al (2007) Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J Exp Med 204(9):2213–2224
Karsunky H et al (2002) Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat Genet 30(3):295–300
Hock H et al (2003) Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 18(1):109–120
Person RE et al (2003) Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat Genet 34(3):308–312
Zhuang D et al (2006) Increased CCAAT enhancer-binding protein epsilon (C/EBPepsilon) expression and premature apoptosis in myeloid cells expressing Gfi-1 N382S mutant associated with severe congenital neutropenia. J Biol Chem 281(16):10745–10751
Zarebski A et al (2008) Mutations in growth factor independent-1 associated with human neutropenia block murine granulopoiesis through colony stimulating factor-1. Immunity 28(3):370–380
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Skokowa J et al (2006) LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nat Med 12(10):1191–1197
Carlsson G et al (2006) Neutrophil elastase and granulocyte colony-stimulating factor receptor mutation analyses and leukemia evolution in severe congenital neutropenia patients belonging to the original Kostmann family in northern Sweden. Haematologica 91(5):589–595
Klein C et al (2007) HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet 39(1):86–92
Suzuki Y et al (1997) HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol 158(6):2736–2744
Gallagher AR et al (2000) The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton. Proc Natl Acad Sci U S A 97(8):4017–4022
Radhika V et al (2004) Galpha13 stimulates cell migration through cortactin-interacting protein Hax-1. J Biol Chem 279(47):49406–49413
Carlsson G, Fasth A (2001) Infantile genetic agranulocytosis, morbus Kostmann: presentation of six cases from the original "Kostmann family" and a review. Acta Paediatr 90(7):757–764
Rezaei N et al (2007) Association of HAX1 deficiency with neurological disorder. Neuropediatrics 38(5):261–263
Matsubara K et al (2007) Severe developmental delay and epilepsy in a Japanese patient with severe congenital neutropenia due to HAX1 deficiency. Haematologica 92(12):e123–e125
Ishikawa N et al (2008) Neurodevelopmental abnormalities associated with severe congenital neutropenia due to the R86X mutation in the HAX1 gene. J Med Genet 45(12):802–807
Germeshausen M et al (2008) Novel HAX1 mutations in patients with severe congenital neutropenia reveal isoform-dependent genotype-phenotype associations. Blood 111(10):4954–4957
Carlsson G et al (2008) Central nervous system involvement in severe congenital neutropenia: neurological and neuropsychological abnormalities associated with specific HAX1 mutations. J Intern Med 264(4):388–400
Zeidler C et al (2009) Clinical implications of ELA2-, HAX1-, and G-CSF-receptor (CSF3R) mutations in severe congenital neutropenia. Br J Haematol 144(4):459–467
de VO, Seynhaeve V (1959) Reticular dysgenesia. Lancet 2(7112):1123–1125
Small TN et al (1999) Association of reticular dysgenesis (thymic alymphoplasia and congenital aleukocytosis) with bilateral sensorineural deafness. J Pediatr 135(3):387–389
Bujan W et al (1993) Effect of recombinant human granulocyte colony-stimulating factor in reticular dysgenesis. Blood 82(5):1684
Pannicke U et al (2009) Reticular dysgenesis (aleukocytosis) is caused by mutations in the gene encoding mitochondrial adenylate kinase 2. Nat Genet 41(1):101–105
Lagresle-Peyrou C et al (2009) Human adenylate kinase 2 deficiency causes a profound hematopoietic defect associated with sensorineural deafness. Nat Genet 41(1):106–111
Lee HJ et al (2007) AK2 activates a novel apoptotic pathway through formation of a complex with FADD and caspase-10. Nat Cell Biol 9(11):1303–1310
Chediak MM (1952) New leukocyte anomaly of constitutional and familial character. Rev Hematol 7(3):362–367
Higashi O (1954) Congenital gigantism of peroxidase granules; the first case ever reported of qualitative abnormity of peroxidase. Tohoku J Exp Med 59(3):315–332
Menasche G et al (2000) Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25(2):173–176
Jung J et al (2006) Identification of a homozygous deletion in the AP3B1 gene causing Hermansky-Pudlak syndrome, type 2. Blood 108(1):362–369
Bohn G et al (2007) A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med 13(1):38–45
Dell'Angelica EC (2007) Bad signals jam organelle traffic. Nat Med 13(1):31–32
Wei ML (2006) Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res 19(1):19–42
Clark RH et al (2003) Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat Immunol 4(11):1111–1120
Huizing M et al (2002) Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2. Pediatr Res 51(2):150–158
Shotelersuk V et al (2000) A new variant of Hermansky-Pudlak syndrome due to mutations in a gene responsible for vesicle formation. Am J Med 108(5):423–427
Kotzot D, Richter K, Gierth-Fiebig K (1994) Oculocutaneous albinism, immunodeficiency, hematological disorders, and minor anomalies: a new autosomal recessive syndrome? Am J Med Genet 50(3):224–227
Enders A et al (2006) Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood 108(1):81–87
Dell'Angelica EC et al (1999) Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol Cell 3(1):11–21
Sugita M et al (2002) Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity 16(5):697–706
Fontana S et al (2006) Innate immunity defects in Hermansky-Pudlak type 2 syndrome. Blood 107(12):4857–4864
Bonifacino JS, Dell'Angelica EC (1999) Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol 145(5):923–926
Wunderlich W et al (2001) A novel 14-kilodalton protein interacts with the mitogen-activated protein kinase scaffold mp1 on a late endosomal/lysosomal compartment. J Cell Biol 152(4):765–776
Kurzbauer R et al (2004) Crystal structure of the p14/MP1 scaffolding complex: how a twin couple attaches mitogen-activated protein kinase signaling to late endosomes. Proc Natl Acad Sci U S A 101(30):10984–10989
Teis D, Wunderlich W, Huber LA (2002) Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell 3(6):803–814
Teis D et al (2006) p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J Cell Biol 175(6):861–868
Melis D et al (2005) Genotype/phenotype correlation in glycogen storage disease type 1b: a multicentre study and review of the literature. Eur J Pediatr 164(8):501–508
Boztug K et al (2009) A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med 360(1):32–43
Bouatia-Naji N et al (2008) A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 320(5879):1085–1088
Chen WM et al (2008) Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest 118(7):2620–2628
Zuelzer WW (1964) Myelokathexis"—a new form of chronic granulocytopenia. Report of a case. N Engl J Med 270:699–704
Gorlin RJ et al (2000) WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet 91(5):368–376
Hernandez PA et al (2003) Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet 34(1):70–74
Sanmun D et al (2006) Stromal-derived factor-1 abolishes constitutive apoptosis of WHIM syndrome neutrophils harbouring a truncating CXCR4 mutation. Br J Haematol 134(6):640–644
Kawai T et al (2007) WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood 109(1):78–84
Balabanian K et al (2005) WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood 105(6):2449–2457
Kostmann R (1956) Infantile genetic agranulocytosis; agranulocytosis infantilis hereditaria. Acta Paediatr Suppl 45(Suppl 105):1–78
Nagata S et al (1986) Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature 319(6052):415–418
Skokowa J et al (2009) NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med 15(2):151–158
Rosenberg PS et al (2006) The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood 107(12):4628–4635
Donini M et al (2007) G-CSF treatment of severe congenital neutropenia reverses neutropenia but does not correct the underlying functional deficiency of the neutrophil in defending against microorganisms. Blood 109(11):4716–4723
Germeshausen M, Ballmaier M, Welte K (2007) Incidence of CSF3R mutations in severe congenital neutropenia and relevance for leukemogenesis: results of a long-term survey. Blood 109(1):93–99
Germeshausen M, Welte K, Ballmaier M (2009) In vivo expansion of cells expressing acquired CSF3R mutations in patients with severe congenital neutropenia. Blood 113(3):668–670
Liu F et al (2008) Csf3r mutations in mice confer a strong clonal HSC advantage via activation of Stat5. J Clin Invest 118(3):946–955
Acknowledgements
We thank all clinical and scientific colleagues referring patients, Dr. Georg Bohn for providing a first draft, Birthe Landes for editorial help. This work was supported by the German José Carreras Foundation, BMBF networks on Congenital bone marrow failure syndromes and Primary immunodeficiency syndromes, and the Clotten Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klein, C., Welte, K. Genetic Insights into Congenital Neutropenia. Clinic Rev Allerg Immunol 38, 68–74 (2010). https://doi.org/10.1007/s12016-009-8130-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-009-8130-5