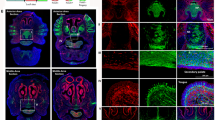
Abstract
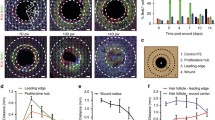
Efficient oral mucosal wound healing requires coordinated responses from epithelial progenitor cells, yet their spatiotemporal recruitment and activation remain unclear. Using a mouse model of palatal mucosal wound healing, we investigated the dynamics of epithelial cells during this process. Proliferation analysis revealed that, in addition to the expected proliferation center near the wound edge, distal cell populations rapidly activated post-injury by elevating their mitotic activity. These distal cells displayed predominant lateral expansion in the basal layer, suggesting roles beyond just tissue renewal. However, while proximal proliferation center cells sustained heightened proliferation until re-epithelialization was completed, distal cells restored basal turnover rates before wound closure, indicating temporally confined contributions. Lineage tracing of Wnt-responsive epithelial cells showed remarkable clone expansion in basal layers both proximally and distally after wounding, contrasting with gradual clone expansion in homeostasis. Although prioritizing tissue repair, epithelial progenitor cells maintained differentiation programs and barrier functions, with the exception of the leading edge. At the leading edge, we found accelerated cell turnover, but the differentiation program was suspended. In summary, our findings uncovered that oral wound re-epithelialization involves two phases: an initial widespread response with proliferation of proximal and distal cells, followed by proliferation confined to the wound proximal region. Uncovering these stage-specific healing mechanisms provides insights for developing targeted therapeutic strategies to improve wound care.
Graphical Abstract







Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary file. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Plikus, M. V., Gay, D. L., Treffeisen, E., Wang, A., Supapannachart, R. J., & Cotsarelis, G. (2012). Epithelial stem cells and implications for wound repair. Seminars in Cell & Developmental Biology, 23(9), 946–953. https://doi.org/10.1016/j.semcdb.2012.10.001
Yang, R., Liu, F., Wang, J., Chen, X., Xie, J., & Xiong, K. (2019). Epidermal stem cells in wound healing and their clinical applications. Stem Cell Research & Therapy, 10(1), 229. https://doi.org/10.1186/s13287-019-1312-z
Mascre, G., et al. (2012). Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature, 489(7415), 257–262. https://doi.org/10.1038/nature11393
Ito, M., et al. (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Medicine, 11(12), 1351–1354. https://doi.org/10.1038/nm1328
Jensen, K. B., et al. (2009). Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell, 4(5), 427–439. https://doi.org/10.1016/j.stem.2009.04.014
Levy, V., Lindon, C., Harfe, B. D., & Morgan, B. A. (2005). Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Developmental Cell, 9(6), 855–861. https://doi.org/10.1016/j.devcel.2005.11.003
Levy, V., Lindon, C., Zheng, Y., Harfe, B. D., & Morgan, B. A. (2007). Epidermal stem cells arise from the hair follicle after wounding. The FASEB Journal, 21(7), 1358–1366. https://doi.org/10.1096/fj.06-6926com
Vagnozzi, A. N., Reiter, J. F., & Wong, S. Y. (2015). Hair follicle and interfollicular epidermal stem cells make varying contributions to wound regeneration. Cell Cycle, 14(21), 3408–3417. https://doi.org/10.1080/15384101.2015.1090062
Bornes, L., Windoffer, R., Leube, R. E., Morgner, J., & van Rheenen, J. (2021). Scratch-induced partial skin wounds re-epithelialize by sheets of independently migrating keratinocytes. Life Science Alliance, 4(1), e202000765. https://doi.org/10.26508/lsa.202000765
Toma, A. I., Fuller, J. M., Willett, N. J., & Goudy, S. L. (2021). Oral wound healing models and emerging regenerative therapies. Translational Research, 236, 17–34. https://doi.org/10.1016/j.trsl.2021.06.003
Leonardo, T. R., Chen, L., & DiPietro, L. A. (2022). Preparation of a murine oral palate wound healing model. STAR Protocols, 3(4), 101727. https://doi.org/10.1016/j.xpro.2022.101727
Byrd, K. M., et al. (2019). Heterogeneity within stratified epithelial stem cell populations maintains the oral mucosa in response to physiological stress. Cell Stem Cell, 25(6), 814-829e6. https://doi.org/10.1016/j.stem.2019.11.005
Yuan, X., Xu, Q., Zhang, X., Van Brunt, L. A., Ticha, P., & Helms, J. A. (2019). Wnt-responsive stem cell fates in the oral mucosa. iScience, 21, 84–94. https://doi.org/10.1016/j.isci.2019.10.016
Blanpain, C., & Fuchs, E. (2009). Epidermal homeostasis: A balancing act of stem cells in the skin. Nature Reviews Molecular Cell Biology, 10(3), 207–217. https://doi.org/10.1038/nrm2636
Haensel, D., et al. (2020). Defining epidermal basal cell states during skin homeostasis and wound healing using single-cell transcriptomics. Cell Reports, 30(11), 3932-3947e6. https://doi.org/10.1016/j.celrep.2020.02.091
Joost, S., et al. (2018). Single-cell transcriptomics of traced epidermal and hair follicle stem cells reveals rapid adaptations during wound healing. Cell Reports, 25(3), 585-597e7. https://doi.org/10.1016/j.celrep.2018.09.059
Aragona, M., et al. (2017). Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nature Communications, 8, 14684. https://doi.org/10.1038/ncomms14684
Dekoninck, S., & Blanpain, C. (2019). Stem cell dynamics, migration and plasticity during wound healing. Nature Cell Biology, 21(1), 18–24. https://doi.org/10.1038/s41556-018-0237-6
Rousselle, P., Braye, F., & Dayan, G. (2019). Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Advanced Drug Delivery Reviews, 146, 344–365. https://doi.org/10.1016/j.addr.2018.06.019
Naik, S., et al. (2017). Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature, 550(7677), 475–480. https://doi.org/10.1038/nature24271
Ordovas-Montanes, J., Beyaz, S., Rakoff-Nahoum, S., & Shalek, A. K. (2020). Distribution and storage of inflammatory memory in barrier tissues. Nature Reviews Immunology, 20(5), 308–320. https://doi.org/10.1038/s41577-019-0263-z
LevraLevron, C., et al. (2023). Tissue memory relies on stem cell priming in distal undamaged areas. Nature Cell Biology, 25(5), 740–753. https://doi.org/10.1038/s41556-023-01120-0
Lyko, F. (2023). Distal memory in wound healing and cancer. Nature Cell Biology, 25(5), 631–632. https://doi.org/10.1038/s41556-023-01132-w
Del Poggetto, E., et al. (2021). Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science, 373(6561), eabj0486. https://doi.org/10.1126/science.abj0486
Yuan, X., Pei, X., Chen, J., Zhao, Y., Brunski, J. B., & Helms, J. A. (2021). Comparative analyses of the soft tissue interfaces around teeth and implants: Insights from a pre-clinical implant model. Journal of Clinical Periodontology, 48(5), 745–753. https://doi.org/10.1111/jcpe.13446
Yuan, X., et al. (2023). Linking the mechanics of chewing to biology of the junctional epithelium. Journal of Dental Research, 102(11), 1252–1260. https://doi.org/10.1177/00220345231185288
Yuan, X., et al. (2020). Formation and regeneration of a Wnt-responsive junctional epithelium. Journal of Clinical Periodontology, 47(12), 1476–1484. https://doi.org/10.1111/jcpe.13371
Yuan, X., Chen, J., Grauer, J. A., Xu, Q., Van Brunt, L. A., & Helms, J. A. (2021). The Junctional Epithelium Is Maintained by a Stem Cell Population. Journal of Dental Research, 100(2), 209–216. https://doi.org/10.1177/0022034520960125
Sun, T., et al. (2020). AXIN2(+) pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell, 26(1), 97-107e6. https://doi.org/10.1016/j.stem.2019.10.011
Salic, A., & Mitchison, T. J. (2008). A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proceedings of the National Academy of Sciences USA, 105(7), 2415–2420. https://doi.org/10.1073/pnas.0712168105
Groeger, S., & Meyle, J. (2019). Oral Mucosal Epithelial Cells. Frontiers in Immunology, 10, 208. https://doi.org/10.3389/fimmu.2019.00208
Squier, C. A., & Kremer, M. J. (2001). Biology of oral mucosa and esophagus. Journal of the National Cancer Institute. Monographs, 29, 7–15. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003443
Sridevi, U., Jain, A., Nagalaxmi, V., Kumar, U. V., & Goyal, S. (2015). Expression of E-cadherin in normal oral mucosa, in oral precancerous lesions and in oral carcinomas. European Journal of Dentistry, 9(3), 364–372. https://doi.org/10.4103/1305-7456.163238
Komori, T., et al. (2018). Type IV collagen alpha6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Scientific Reports, 8(1), 2612. https://doi.org/10.1038/s41598-018-21000-0
Murakami, H., Okamura, K., Aoki, S., Sakagami, R., & Yamazaki, J. (2014). Association of caspase-14 and filaggrin expression with keratinization of the oral mucosa and reconstruction culture rat models. Journal of Periodontal Research, 49(6), 703–710. https://doi.org/10.1111/jre.12152
Smith, S. A., & Dale, B. A. (1986). Immunologic localization of filaggrin in human oral epithelia and correlation with keratinization. The Journal of Investigative Dermatology, 86(2), 168–172. https://doi.org/10.1111/1523-1747.ep12284213
Leung, E. Y., et al. (2017). DNA damage marker phosphorylated histone H2AX is a potential predictive marker for progression of epithelial dysplasia of the oral cavity. Histopathology, 71(4), 522–528. https://doi.org/10.1111/his.13260
Siddiqui, M. S., Francois, M., Fenech, M. F., & Leifert, W. R. (2015). gammaH2AX responses in human buccal cells exposed to ionizing radiation. Cytometry Part A, 87(4), 296–308. https://doi.org/10.1002/cyto.a.22607
Park, S., et al. (2017). Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nature Cell Biology, 19(2), 155–163. https://doi.org/10.1038/ncb3472
Singh, G., & Chanda, A. (2021). Mechanical properties of whole-body soft human tissues: a review. Biomedical Materials, 16(6), 062004. https://doi.org/10.1088/1748-605X/ac2b7a
Dutzan, N., et al. (2017). On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity, 46(1), 133–147. https://doi.org/10.1016/j.immuni.2016.12.010
Boyle, C. J., et al. (2019). Morphology and composition play distinct and complementary roles in the tolerance of plantar skin to mechanical load. Sci Adv, 5(10), eaay0244. https://doi.org/10.1126/sciadv.aay0244
Naik, S., & Fuchs, E. (2022). Inflammatory memory and tissue adaptation in sickness and in health. Nature, 607(7918), 249–255. https://doi.org/10.1038/s41586-022-04919-3
Niethammer, P. (2016). The early wound signals. Current Opinion in Genetics & Development, 40, 17–22. https://doi.org/10.1016/j.gde.2016.05.001
Enyedi, B., & Niethammer, P. (2015). Mechanisms of epithelial wound detection. Trends in Cell Biology, 25(7), 398–407. https://doi.org/10.1016/j.tcb.2015.02.007
Dunnill, C., et al. (2017). Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. International Wound Journal, 14(1), 89–96. https://doi.org/10.1111/iwj.12557
Author information
Authors and Affiliations
Contributions
T.T., S.F., D.O., T.Z.: Contributed to data acquisition and interpretation, performed statistical analyses, and drafted and critically revised the manuscript. X.Y.: Contributed to the conception and design of the research, data acquisition, data interpretation, and drafted and critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical Approval
All animal experimental protocols were approved by the Indiana University Institutional Animal Care and Use Committee (#21030).
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thompson, T., Flanagan, S., Ortega-Gonzalez, D. et al. Immediate but Temporal Response: The Role of Distal Epithelial Cells in Wound Healing. Stem Cell Rev and Rep (2024). https://doi.org/10.1007/s12015-024-10734-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12015-024-10734-2