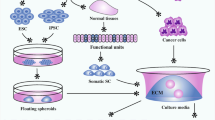
Abstract
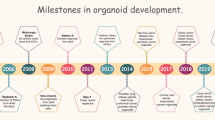
In recent years, the rapid emergence of 3D organoid technology has garnered significant attention from researchers. These miniature models accurately replicate the structure and function of human tissues and organs, offering more physiologically relevant platforms for cancer research. These intricate 3D structures not only serve as promising models for studying human cancer, but also significantly contribute to the advancement of various potential applications in the field of cancer research. To date, organoids have been efficiently constructed from both normal and malignant tissues originating from patients. Using such bioengineering platforms, simulations of infections and cancer processes, mutations and carcinogenesis can be achieved, and organoid technology is also expected to facilitate drug testing and personalized therapies. In conclusion, regenerative medicine has the potential to enhance organoid technology and current transplantation treatments by utilizing genetically identical healthy organoids as substitutes for irreversibly deteriorating diseased organs. This review explored the evolution of cancer organoids and emphasized the significant role these models play in fundamental research and the advancement of personalized medicine in oncology.
Graphical Abstract





Similar content being viewed by others
Data Availability
All required data included in text and supplementary. Any further any formation required is available with the corresponding author.
Code Availability
All required code included in text and supplementary.
Abbreviations
- MRA:
-
Microraft array
- HCS:
-
High-content screening
- CF:
-
Cystic fibrosis
- ESCs:
-
Embryonic stem cells
- iPSC:
-
Induced pluripotent stem cells
- Rb:
-
Retinoblastoma
- PSCs:
-
Pluripotent stem cells
- hPSCs:
-
Human pluripotent stem cells
- SAM:
-
S-adenosylmethionine
- PM:
-
Paraxial mesoderm
- WOI:
-
Window-of-implantation
- CRC:
-
Colorectal cancer
- PDOs:
-
Patient-derived organoids
- OC:
-
Ovarian cancer
- HGSOC:
-
High-grade serous ovarian cancer
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PRMT5:
-
Protein arginine methyltransferase gene 5
- PDXs:
-
Patient-derived xenografts
- DLA:
-
Diagnostic leukapheresis
- CTC:
-
Circulating tumor cell
- NSCLC:
-
Non-small cell lung cancer
References
Xu, H., Lyu, X., Yi, M., Zhao, W., Song, Y., & Wu, K. (2018). Organoid technology and applications in cancer research. Journal of Hematology & Oncology, 11(1). https://doi.org/10.1186/s13045-018-0662-9. [online].
Clevers, H., & Tuveson, D. A. (2019). Organoid models for cancer research. Annual Review of Cancer Biology, 3(1), 223–234. https://doi.org/10.1146/annurev-cancerbio-030518-055702
Yang, L., Yang, S., Li, X., Li, B., Li, Y., Zhang, X., Ma, Y., Peng, X., Jin, H., Fan, Q., Wei, S., Liu, J., & Li, H. (2019). Tumor organoids: From inception to future in cancer research. Cancer Letters, 454, 120–133. https://doi.org/10.1016/j.canlet.2019.04.005
Liu, C., Qin, T., Huang, Y., Li, Y., Chen, G., & Sun, C. (2020). Drug screening model meets cancer organoid technology. Translational Oncology, 13(11). https://doi.org/10.1016/j.tranon.2020.100840. [online].
Kretzschmar, K. (2020). Cancer research using organoid technology. Journal of Molecular Medicine. https://doi.org/10.1007/s00109-020-01990-z
Gilazieva, Z., Ponomarev, A., Rutland, C., Rizvanov, A., & Solovyeva, V. (2020). Promising applications of tumor spheroids and organoids for personalized medicine. Cancers, 12(10), 2727. https://doi.org/10.3390/cancers12102727
Mullenders, J., et al. (2019). Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proceedings of the National Academy of Sciences, 116(10), 4567–4574. https://www.pnas.org/content/116/10/4567. https://doi.org/10.1073/pnas.1803595116. Accessed 20 Dec 2023
Lensink, M. A., Boers, S. N., Jongsma, K. R., Carter, S. E., van der Ent, C. K., & Bredenoord, A. L. (2021). Organoids for personalized treatment of Cystic Fibrosis: Professional perspectives on the ethics and governance of organoid biobanking. Journal of Cystic Fibrosis, 20(3), 443–451. https://doi.org/10.1016/j.jcf.2020.11.015
Kim, S. K., Kim, Y. H., Park, S., & Cho, S.-W. (2021). Organoid engineering with microfluidics and biomaterials for liver, lung disease, and cancer modeling. Acta Biomaterialia, 132, 37–51. https://doi.org/10.1016/j.actbio.2021.03.002. [online].
Yang, H., Wang, Y., Wang, P., Zhang, N., & Wang, P. (2021). Tumor organoids for cancer research and personalized medicine. Cancer Biology and Medicine, 18(-). https://doi.org/10.20892/j.issn.2095-3941.2021.0335
Olayanju, A., Jones, L., Greco, K., Goldring, C. E., & Ansari, T. (2018). Application of porcine gastrointestinal organoid units as a potential in vitro tool for drug discovery and development. Journal of Applied Toxicology, 39(1), 4–15. https://doi.org/10.1002/jat.3641
Cruz-Acuña, R., & García, A. J. (2019). Engineered materials to model human intestinal development and cancer using organoids. Experimental Cell Research, 377(1–2), 109–114. https://doi.org/10.1016/j.yexcr.2019.02.017
Liu, H., Zhang, Y., Zhang, Y.-Y., Li, Y.-P., Hua, Z.-Q., Zhang, C.-J., Wu, K.-C., Yu, F., Zhang, Y., Su, J., & Jin, Z.-B. (2020). Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proceedings of the National Academy of Sciences of the United States of America, 117(52), 33628–33638. https://doi.org/10.1073/pnas.2011780117. [online].
Mullenders, J., Buijs, A., Fielmich, L.-E., Sluimer, J., Sun, J., Vries, R. G., & Boj, S. F. (2021). Abstract 126: HUB Organoids: Bringing the ‘patient in the lab’ for preclinical and clinical development. Cancer Research, 81(13_Supplement), 126–126. https://doi.org/10.1158/1538-7445.am2021-126
Duzagac, F., Saorin, G., Memeo, L., Canzonieri, V., & Rizzolio, F. (2021). Microfluidic organoids-on-a-chip: Quantum leap in cancer research. Cancers, 13(4), 737. https://doi.org/10.3390/cancers13040737. [online].
Qu, J., Kalyani, F. S., Liu, L., Cheng, T., & Chen, L. (2021). Tumor organoids: Synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Communications, 41(12), 1331–1353. https://doi.org/10.1002/cac2.12224
Uzquiano, A., Kedaigle, A. J., Pigoni, M., Paulsen, B., Adiconis, X., Kim, K., Faits, T., Nagaraja, S., Antón-Bolaños, N., Gerhardinger, C., Tucewicz, A., Murray, E., Jin, X., Buenrostro, J., Chen, F., Velasco, S., Regev, A., Levin, J. Z., & Arlotta, P. (2022). Proper acquisition of cell class identity in organoids allows definition of fate specification programs of the human cerebral cortex. Cell, 185(20), 3770-3788.e27. https://doi.org/10.1016/j.cell.2022.09.010. [online].
Richiardone, E., Van den Bossche, V., & Corbet, C. (2022). Metabolic studies in organoids: Current applications. Opportunities and Challenges. Organoids, 1(1), 85–105. https://doi.org/10.3390/organoids1010008
Nuciforo, S., Fofana, I., Matter, M. S., Blumer, T., Calabrese, D., Boldanova, T., Piscuoglio, S., Wieland, S., Ringnalda, F., Schwank, G., Terracciano, L. M., Ng, C. K. Y., & Heim, M. H. (2018). Organoid models of human liver cancers derived from tumor needle biopsies. Cell Reports, 24(5), 1363–1376. https://doi.org/10.1016/j.celrep.2018.07.001.PMID:30067989;PMCID:PMC6088153
Augustyniak, J., Bertero, A., Coccini, T., Baderna, D., Buzanska, L., & Caloni, F. (2019). Organoids are promising tools for species-specific in vitro toxicological studies. Journal of Applied Toxicology. https://doi.org/10.1002/jat.3815
Farzaneh, Z., Abbasalizadeh, S., Asghari-Vostikolaee, M.-H., Alikhani, M., Joaquim & Baharvand, H. (2020). Dissolved oxygen concentration regulates human hepatic organoid formation from pluripotent stem cells in a fully controlled bioreactor. Biotechnology and Bioengineering, 117(12), 3739–3756. https://doi.org/10.1002/bit.27521
Akiva, A., Melke, J., Ansari, S., Liv, N., Meijden, R., Erp, M., Zhao, F., Stout, M., Nijhuis, W. H., Heus, C., Muñiz Ortera, C., Fermie, J., Klumperman, J., Ito, K., Sommerdijk, N., & Hofmann, S. (2021). An organoid for woven bone. Advanced Functional Materials, 31(17), 2010524. https://doi.org/10.1002/adfm.202010524
Wang, Q., Xiong, Y., Zhang, S., Sui, Y., Yu, C., Liu, P., Li, H., Guo, W., Gao, Y., Przepiorski, A., Davidson, A. J., Guo, M., & Zhang, X. (2021). The dynamics of metabolic characterization in iPSC-derived kidney organoid differentiation via a comparative omics approach. Frontiers in Genetics, 12, 632810. https://doi.org/10.3389/fgene.2021.632810. [online].
Zahmatkesh, E., Khoshdel-Rad, N., Mirzaei, H., Shpichka, A., Timashev, P., Mahmoudi, T., & Vosough, M. (2021). Evolution of organoid technology: Lessons learnt in co-culture systems from developmental biology. Developmental Biology, 475, 37–53. https://doi.org/10.1016/j.ydbio.2021.03.001
Tortorella, I., Argentati, C., Emiliani, C., Martino, S., & Morena, F. (2021). The role of physical cues in the development of stem cell-derived organoids. European Biophysics Journal, 51(2), 105–117. https://doi.org/10.1007/s00249-021-01551-3
Gjorevski, N., Nikolaev, M., Brown, T.E., Mitrofanova, O., Brandenberg, N., DelRio, F.W., Yavitt, F.M., Liberali, P., Anseth, K.S., & Lutolf, M.P. (2022). Tissue geometry drives deterministic organoid patterning. Science, 375(6576). https://doi.org/10.1126/science.aaw9021
Budjan, C., Liu, S., Ranga, A., Gayen, S., Pourquié, O., & Hormoz, S. (n.d.). Paraxial mesoderm organoids model development of human somites. eLife, 11, e68925. https://doi.org/10.7554/eLife.68925. [online].
Yu, Z., Zhao, R.-S., Yang, C., Song, J., Liu, P., Li, Y., Liu, B., Li, T., Yin, C., Lü, M., Hou, Z., Zhang, C., Chen, Z., Wu, K., & Han, Z. (2023). Human receptive endometrial organoid for deciphering the implantation window. bioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2023.07.27.550771
Arena, S., Corti, G., Durinikova, E., Montone, M., Reilly, N., Russo, M., Lorenzato, A., Arcella, P., Lazzari, L., Rospo, G., Pagani, M., Cancelliere, C., Negrino, C., Isella, C., Bartolini, A., Cassingena, A., Sartore-Bianchi, A., Mauri, G., Bianchi, P., & Mittica, G. (2020). A subset of colorectal cancers with cross-sensitivity to olaparib and oxaliplatin. 26(6), 1372–1384. https://doi.org/10.1158/1078-0432.ccr-19-2409
Wong, C., Han, H.-W., Tien, Y., & Hsu, S. (2019). Biomaterial substrate-derived compact cellular spheroids mimicking the behavior of pancreatic cancer and microenvironment. Biomaterials, 213, 119202–119202. https://doi.org/10.1016/j.biomaterials.2019.05.013
Kondo, J., & Inoue, M. (2019). Application of cancer organoid model for drug screening and personalized therapy. Cells, 8(5), 470. https://doi.org/10.3390/cells8050470. [online].
Luo, Z., Zhou, X., Mandal, K., He, N., Wennerberg, W., Qu, M., Jiang, X., Sun, W., & Khademhosseini, A. (2021). Reconstructing the tumor architecture into organoids. Advanced Drug Delivery Reviews, 176, 113839–113839. https://doi.org/10.1016/j.addr.2021.113839
Ren, X., Chen, W., Yang, Q., Li, X., & Xu, L. (2022). Patient-derived cancer organoids for drug screening: Basic technology and clinical application. Journal of Gastroenterology and Hepatology, 37(8), 1446–1454. https://doi.org/10.1111/jgh.15930
Xie, X., Li, X. and Song, W. (2023). Tumor organoid biobank-new platform for medical research. Scientific Reports, 13(1). https://doi.org/10.1038/s41598-023-29065-2
Zhao, D.-K., Liang, J., Huang, X., Shen, S. and Wang, J. (2023). Organoids technology for advancing the clinical translation of cancer nanomedicine. WIREs Nanomedicine and Nanobiotechnology, 15(5). https://doi.org/10.1002/wnan.1892
El-Salam, M. A., Troulis, M. J., Pan, C., & Rao, R. (2023). Unlocking the potential of organoids in cancer treatment and translational research: An application of cytologic techniques. Cancer Cytopathology. https://doi.org/10.1002/cncy.22769
Kopper, O., de Witte, C. J., Lõhmussaar, K., Valle-Inclan, J. E., Hami, N., Kester, L., Balgobind, A. V., Korving, J., Proost, N., Begthel, H., van Wijk, L. M., Revilla, S. A., Theeuwsen, R., van de Ven, M., van Roosmalen, M. J., Ponsioen, B., Ho, V. W. H., Neel, B. G., Bosse, T., & Gaarenstroom, K. N. (2019). An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nature Medicine, 25(5), 838–849. https://doi.org/10.1038/s41591-019-0422-6. [online].
Maenhoudt, N., Defraye, C., Boretto, M., Jan, Z., Heremans, R., Boeckx, B., Hermans, F., Arijs, I., Cox, B., Nieuwenhuysen, E. V., Vergote, I., Rompuy, A.-S.V., Lambrechts, D., Timmerman, D., & Vankelecom, H. (2020). Developing organoids from ovarian cancer as experimental and preclinical models. Stem Cell Reports, 14(4), 717–729. https://doi.org/10.1016/j.stemcr.2020.03.004. [online].
Witte, C.J. de, Valle-Inclan, J.E., Hami, N., Lõhmussaar, K., Kopper, O., Vreuls, C.P.H., Jonges, G.N., Diest, P. van, Nguyen, L., Clevers, H., Kloosterman, W.P., Cuppen, E., Snippert, H.J.G., Zweemer, R.P., Witteveen, P.O., & Stelloo, E. (2020). Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Reports, 31(11). https://doi.org/10.1016/j.celrep.2020.107762. [online].
Bi, J., Thiel, K. W., Litman, J. M., Zhang, Y., Devor, E. J., Newtson, A. M., Schnieders, M. J., Bosquet, J. G., & Leslie, K. K. (2020). Characterization of a TP53 somatic variant of unknown function from an ovarian cancer patient using organoid culture and computational modeling. Clinical Obstetrics and Gynecology, 63(1), 109–119. https://doi.org/10.1097/grf.0000000000000516
Lui, G. Y. L., Richardson, A. B., Chatterjee, P., Pollastro, M., Lints, M., Peretti, D., Rosati, R., Appleyard, L., Durenberger, G., Diaz, R. L., Gurley, K. E., Stork, I. N., Whitney, A., Kapeli, K., Swan, H. A., Memari, Y., Davies, H., Nik-Zainal, S., Banda, K., & Gray, H. J. (2021). Abstract 534: Functional drug screening of organoids from ovarian cancer patients demonstrates clinical and genomic concordance and identifies novel therapeutic vulnerabilities. Clinical Research (Excluding Clinical Trials). https://doi.org/10.1158/1538-7445.am2021-534
Maru, Y., Tanaka, N., Itami, M., & Hippo, Y. (2019). Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecologic Oncology, 154(1), 189–198. https://doi.org/10.1016/j.ygyno.2019.05.005
Battaglia, A., Piermattei, A., Buzzonetti, A., Pasciuto, T., Zampetti, N., Fossati, M., Angelico, G., Iacobelli, V., Nero, C., Iannucci, V., Scambia, G., Fagotti, A., & Fattorossi, A. (2021). PD-L1 expression on circulating tumour-derived microvesicles as a complementary tool for stratification of high-grade serous ovarian cancer patients. Cancers, 13(20), 5200–5200. https://doi.org/10.3390/cancers13205200
Rauth, S., et al. (2021). Recent advances in organoid development and applications in disease modeling. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1875(2), 188527. https://www.sciencedirect.com/science/article/pii/S0304419X21000263. https://doi.org/10.1016/j.bbcan.2021.188527. Accessed 5 Jan 2024
Saito, Y. (2019). Establishment of an organoid bank of biliary tract and pancreatic cancers and its application for personalized therapy and future treatment. Journal of Gastroenterology and Hepatology, 34(11), 1906–1910. https://doi.org/10.1111/jgh.14773
Camara, R., Ogbeni, D., Gerstmann, L., Ostovar, M., Hurer, E., Scott, M., Mahmoud, N. G., Radon, T., Crnogorac-Jurcevic, T., Patel, P., Mackenzie, L. S., Chau, D. Y. S., Kirton, S. B., & Rossiter, S. (2020). Discovery of novel small molecule inhibitors of S100P with in vitro anti-metastatic effects on pancreatic cancer cells. European Journal of Medicinal Chemistry, 203, 112621. https://doi.org/10.1016/j.ejmech.2020.112621. [online].
Wei, X., Yang, J., Adair, S. J., Ozturk, H., Kuscu, C., Lee, K. Y., Kane, W. J., O’Hara, P. E., Liu, D., Demirlenk, Y. M., Habieb, A. H., Yilmaz, E., Dutta, A., Bauer, T. W., & Adli, M. (2020). Targeted CRISPR screening identifies PRMT5 as synthetic lethality combinatorial target with gemcitabine in pancreatic cancer cells. Proceedings of the National Academy of Sciences, 117(45), 28068–28079. https://doi.org/10.1073/pnas.2009899117. [online].
Driehuis, E., Gracanin, A., Vries, R. G. J., Clevers, H., & Boj, S. F. (2020). Establishment of Pancreatic Organoids from Normal Tissue and Tumors. STAR Protocols, 1(3), 100192. https://doi.org/10.1016/j.xpro.2020.100192. [online].
Bengtsson, A., Andersson, R., Rahm, J., Ganganna, K., Andersson, B., & Ansari, D. (2021). Organoid technology for personalized pancreatic cancer therapy. Cellular Oncology, 44(2), 251–260. https://doi.org/10.1007/s13402-021-00585-1
Yang, G., Guan, W., Cao, Z., Guo, W., Xiong, G., Zhao, F., Feng, M., Qiu, J., Liu, Y., Zhang, M. Q., You, L., Zhang, T., Zhao, Y., & Gu, J. (2021). Integrative genomic analysis of gemcitabine resistance in pancreatic cancer by patient-derived xenograft models. Clinical Cancer Research, 27(12), 3383–3396. https://doi.org/10.1158/1078-0432.ccr-19-3975. [online].
Chin, Y. T., He, Z.-R., Chen, C., Chu, H.-C., Ho, Y., Su, P.-Y., Yang, Y., Wang, K., Shih, Y.-J., Chen, Y. R., Pedersen, J. Z., Incerpi, S., Nana, A. W., Tang, H.-Y., Lin, H.-Y., Mousa, S. A., Davis, P. J., & Whang‐Peng, J. (2019). Tetrac and NDAT induce anti-proliferation via integrin αvβ3 in colorectal cancers with different K-RAS status. Frontiers in Endocrinology, 10. https://doi.org/10.3389/fendo.2019.00130
Wang, Y., Liao, H., Zheng, T., Wang, J., Guo, D., Lü, Z., Li, Z., Chen, Y., Shen, L., Zhang, Y., & Gao, J. (2020). Conditionally reprogrammed colorectal cancer cells combined with mouse avatars identify synergy between EGFR and MEK or CDK4/6 inhibitors. PubMed, 10(1), 249–262.
Yao, Y., et al. (2020). Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell, 26(1), 17-26.e6. https://doi.org/10.1016/j.stem.2019.10.010
Smit, T., Calitz, C., Willers, C., Svitina, H., Hamman, J. H., Fey, S. J., Gouws, C., & Wrzesinski, K. (2020). Characterization of an alginate encapsulated LS180 spheroid model for anti-colorectal cancer compound screening. ACS Medicinal Chemistry Letters, 11(5), 1014–1021. https://doi.org/10.1021/acsmedchemlett.0c00076
Ramzy, G. M., Koessler, T., Ducrey, E., McKee, T., Ris, F., Buchs, N., Rubbia-Brandt, L., Dietrich, P.-Y., & Nowak-Sliwinska, P. (2020). Patient-derived in vitro models for drug discovery in colorectal carcinoma. Cancers, 12(6), 1423. https://doi.org/10.3390/cancers12061423. [online].
Mout, L., van Dessel, Kraan, J., Jong, Neves, R. P., Erkens-Schulze, S., Beaufort, C. M., Sieuwerts, A. M., van Riet, J., Woo, T., de Wit, R., Sleijfer, S., Hamberg, P., Sandberg, Y., te Boekhorst, P., Martens, J. W. M., Stoecklein, N. H., van Weerden & Lolkema, M. P. (2021). Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. European Journal of Cancer, 150, 179–189. https://doi.org/10.1016/j.ejca.2021.03.023
(2018). Metastatic prostate cancer. New England Journal of Medicine, 378(17), 1653–1654. https://doi.org/10.1056/nejmc1803343
Wu, Q., Wei, X., Pan, Y., Zou, Y., Hu, N., & Wang, P. (2018). Bionic 3D spheroids biosensor chips for high-throughput and dynamic drug screening. 20(4). https://doi.org/10.1007/s10544-018-0329-x
Sachs, N., Papaspyropoulos, A., Zomer-van Ommen, D. D., Heo, I., Böttinger, L., Klay, D., Weeber, F., Huelsz-Prince, G., Iakobachvili, N., Amatngalim, G. D., de Ligt, J., van Hoeck, A., Proost, N., Viveen, M. C., Lyubimova, A., Teeven, L., Derakhshan, S., Korving, J., Begthel, H., & Dekkers, J. F. (2019). Long-term expanding human airway organoids for disease modeling. The EMBO journal, 38(4). https://doi.org/10.15252/embj.2018100300. [online].
Mazzocchi, A., Devarasetty, M., Herberg, S., Petty, W. J., Marini, F., Miller, L., Kucera, G., Dukes, D. K., Ruiz, J., Skardal, A., & Soker, S. (2019). Pleural effusion aspirate for use in 3D lung cancer modeling and chemotherapy screening. ACS Biomaterials Science & Engineering, 5(4), 1937–1943. https://doi.org/10.1021/acsbiomaterials.8b01356
Kim, M., Mun, H., Sung, C. O., Cho, E. J., Jeon, H.-J., Chun, S.-M., Jung, D. J., Shin, T. H., Jeong, G. S., Kim, D. K., Choi, E. K., Jeong, S.-Y., Taylor, A. M., Jain, S., Meyerson, M., & Jang, S. J. (2019). Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nature Communications, 10(1), 3991. https://doi.org/10.1038/s41467-019-11867-6. [online].
Pauli, C., Puca, L., Emerling, B. M., Hopkins, B., Sboner, A., Elemento, O., Mosquera, J. M., Beltran, H., & Rubin, M. A. (2016). Abstract 619: Personalized models to guide precision medicine. Cancer Research, 76(14_Supplement), 619–619. https://doi.org/10.1158/1538-7445.am2016-619
Ho, B., Pek, N., & Soh, B.-S. (2018). Disease modeling using 3D organoids derived from human induced pluripotent stem cells. International Journal of Molecular Sciences, 19(4), 936. https://doi.org/10.3390/ijms19040936
Lau, H. C. H., Kranenburg, O., Xiao, H., & Yu, J. (2020). Organoid models of gastrointestinal cancers in basic and translational research. Nature Reviews Gastroenterology & Hepatology, 17(4), 203–222. https://doi.org/10.1038/s41575-019-0255-2
Horie, M., et al. (2012). Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochemical and Biophysical Research Communications, 423(1), 158–163. https://doi.org/10.1016/j.bbrc.2012.05.104
Salgueiredo-Giudice, F., et al. (2012). An in vitro study showing the three-dimensional microenvironment influence over the behavior of head and neck squamous cell carcinoma. Medicina Oral Patologia Oral Y Cirugia Bucal, e377–e382. https://doi.org/10.4317/medoral.17538. Accessed 26 Feb. 2024.
Maru, Y., et al. (2019). Establishment and characterization of patient‐derived organoids from a young patient with cervical clear cell carcinoma. Cancer Science, 110(9), 2992–3005. https://www.onlinelibrary.wiley.com/doi/abs/10.1111/cas.14119. https://doi.org/10.1111/cas.14119. Accessed 6 Jan 2024
Velasco, S, et al. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature, 570(7762), 523–527. https://www.ncbi.nlm.nih.gov/pubmed/31168097. https://doi.org/10.1038/s41586-019-1289-x. Accessed 6 Jan 2024
Acknowledgements
This work was supported by Hebei Medical University and funded by National Natural Science Foundation of China (No. 81971474, 8197061369 and 82201953), General Program of China Postdoctoral Science Foundation (No. 2021M701036), Hebei Key R&D Program Project Special Project for the Construction of Beijing-Tianjin-Hebei Collaborative Innovation Community (No. 22347702D), and Key Project of Natural Science Foundation of Hebei Province (C2021206011).
Funding
The work was supported by grants from the National Natural Science Foundation of China (No. 81971474, 8197061369 and 82201953), General Program of China Postdoctoral Science Foundation (No. 2021M701036), Hebei Key R&D Program Project Special Project for the Construction of Beijing-Tianjin-Hebei Collaborative Innovation Community (No. 22347702D), and Key Project of Natural Science Foundation of Hebei Province (C2021206011).
Author information
Authors and Affiliations
Contributions
YJY performed the selection of literature, drafted the manuscript, and prepared the figures. YJY, JLC, YJK, and ZJG collected the related references. YH and YJY carried out the design of this review and revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors have agreed to publish this manuscript.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Kong, Y., Cui, J. et al. Advances and Applications of Cancer Organoids in Drug Screening and Personalized Medicine. Stem Cell Rev and Rep (2024). https://doi.org/10.1007/s12015-024-10714-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s12015-024-10714-6