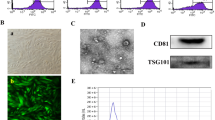
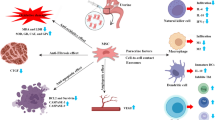
Abstract
Background
Female reproductive disorders, such as premature ovarian insufficiency (POI), intrauterine adhesion (IUA) or thin endometrium, and polycystic ovary syndrome (PCOS), are the main factors affecting fertility. Mesenchymal stem cells derived–extracellular vesicles (MSC-EVs) have gained traction as a new potential treatment and were widely studied in these diseases. However, their impact is still not fully clear.
Methods
A systematic search of PubMed, Web of Science, EMBASE, the Chinese National Knowledge of Infrastructure, and WanFang online databases was performed up to September 27th, 2022, and the studies of MSC-EVs-based therapy on the animal models of female reproductive diseases were included. The primary outcomes were anti-Müllerian hormone (AMH) in POI and endometrial thickness in IUA, respectively.
Results
28 studies (POI, N = 15; IUA, N = 13) were included. For POI, MSC-EVs improved AMH at 2 weeks (SMD 3.40, 95% CI 2.02 to 4.77) and 4 weeks (SMD 5.39, 95% CI 3.43 to 7.36) compared with placebo, and no difference was found when compared with MSCs in AMH (SMD -2.03, 95% CI -4.25 to 0.18). For IUA, MSC-EVs treatment could increase the endometrial thickness at 2 weeks (WMD 132.36, 95% CI 118.99 to 145.74), but no improvement was found at 4 weeks (WMD 166.18, 95% CI -21.44 to 353.79). The combination of MSC-EVs with hyaluronic acid or collagen had a better effect on the endometrial thickness (WMD 105.31, 95% CI 85.49 to 125.13) and glands (WMD 8.74, 95% CI 1.34 to 16.15) than MSC-EVs alone. The medium dose of EVs may allow for great benefits in both POI and IUA.
Conclusions
MSC-EVs treatment could improve the functional and structural outcomes in female reproductive disorders. The combination of MSC-EVs with HA or collagen may enhance the effect. These findings can accelerate the translation of MSC-EVs treatment to human clinical trials
Graphical Abstract







Similar content being viewed by others
Data Availability
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Change history
07 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12015-023-10624-z
References
Vander Borght, M., & Wyns, C. (2018). Fertility and infertility: Definition and epidemiology. Clinical Biochemistry, 62, 2–10. https://doi.org/10.1016/j.clinbiochem.2018.03.012
Ishizuka, B. (2021). Current Understanding of the Etiology, Symptomatology, and Treatment Options in Premature Ovarian Insufficiency (POI). Frontiers In Endocrinology, 12, 626924. https://doi.org/10.3389/fendo.2021.626924
Salazar, C. A., Isaacson, K., & Morris, S. (2017). A comprehensive review of Asherman’s syndrome: Causes, symptoms and treatment options. Current Opinion In Obstetrics & Gynecology, 29(4), 249–256. https://doi.org/10.1097/GCO.0000000000000378
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., & Perez-Fernandez, R. (2017). Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. International Journal of Molecular Science, 18(9), 1852. https://doi.org/10.3390/ijms18091852
Ding, D.-C., Shyu, W.-C., & Lin, S.-Z. (2011). Mesenchymal stem cells. Cell Transplanation, 20(1), 5–14. https://doi.org/10.3727/096368910X
Ullah, I., Subbarao, R. B., & Rho, G. J. (2015). Human mesenchymal stem cells - current trends and future prospective. Bioscience Reports, 35(2), e00191. https://doi.org/10.1042/BSR20150025
Saha, S., Roy, P., Corbitt, C., & Kakar, S. S. (2021). Application of Stem Cell Therapy for Infertility. Cells, 10(7), 1613. https://doi.org/10.3390/cells10071613
Esfandyari, S., Chugh, R. M., Park, H.-S., Hobeika, E., Ulin, M., et al. (2020). Mesenchymal Stem Cells as a Bio Organ for Treatment of Female Infertility. Cells, 9(10), 2253. https://doi.org/10.3390/cells9102253
Chen, L., Guo, S., Wei, C., Li, H., Wang, H., et al. (2018). Effect of stem cell transplantation of premature ovarian failure in animal models and patients: A meta-analysis and case report. Experimental and Therapeutic Medicine, 15(5), 4105–4118. https://doi.org/10.3892/etm.2018.5970
Bhartiya, D., Singh, P., Sharma, D., & Kaushik, A. (2022). Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues. Stem Cell Reviews and Reports, 18(5), 1718–1727. https://doi.org/10.1007/s12015-021-10243-6
Mendt, M., Rezvani, K., & Shpall, E. (2019). Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplantation, 54(Suppl 2), 789–792. https://doi.org/10.1038/s41409-019-0616-z
El Andaloussi, S., Mäger, I., Breakefield, X. O., & Wood, M. J. A. (2013). Extracellular vesicles: Biology and emerging therapeutic opportunities Nature Reviews. Drug Discovery, 12(5), 347–357. https://doi.org/10.1038/nrd3978
Lai, C.P.-K., & Breakefield, X. O. (2012). Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Frontiers In Physiology, 3, 228. https://doi.org/10.3389/fphys.2012.00228
Neven, K. Y., Nawrot, T. S., & Bollati, V. (2017). Extracellular Vesicles: How the External and Internal Environment Can Shape Cell-To-Cell Communication. Current Environmental Health Reports, 4(1), 30–37. https://doi.org/10.1007/s40572-017-0130-7
Keshtkar, S., Azarpira, N., & Ghahremani, M. H. (2018). Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Research & Therapy, 9(1), 63. https://doi.org/10.1186/s13287-018-0791-7
Zhao, A. G., Shah, K., Cromer, B., & Sumer, H. (2020). Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem Cells International, 2020, 8825771. https://doi.org/10.1155/2020/8825771
Liao, Z., Liu, C., Wang, L., Sui, C., & Zhang, H. (2021). Therapeutic Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Female Reproductive Diseases. Frontiers In Endocrinology, 12, 665645. https://doi.org/10.3389/fendo.2021.665645
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research Ed.), 339, b2535. https://doi.org/10.1136/bmj.b2535
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research ed.), 372, n71. https://doi.org/10.1136/bmj.n71
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. https://doi.org/10.1080/20013078.2018.1535750
Tieu, A., Lalu, M. M., Slobodian, M., Gnyra, C., Fergusson, D. A., et al. (2020). An Analysis of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Preclinical Use. ACS Nano, 14(8), 9728–9743. https://doi.org/10.1021/acsnano.0c01363
Hooijmans, C. R., Rovers, M. M., de Vries, R. B. M., Leenaars, M., Ritskes-Hoitinga, M., et al. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Medical Research Methodology, 14, 43. https://doi.org/10.1186/1471-2288-14-43
Fergusson, D. A., Avey, M. T., Barron, C. C., Bocock, M., Biefer, K. E., et al. (2019). Reporting preclinical anesthesia study (REPEAT): Evaluating the quality of reporting in the preclinical anesthesiology literature. PLoS One, 14(5), e0215221. https://doi.org/10.1371/journal.pone.0215221
Yang, M., Lin, L., Sha, C., Li, T., Zhao, D., et al. (2020). Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Laboratory Investigation, 100(3), 342–352. https://doi.org/10.1038/s41374-019-0321-y
Zhang, S., Huang, B., Su, P., Chang, Q., Li, P., et al. (2021). Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Research & Therapy, 12(1), 178. https://doi.org/10.1186/s13287-021-02255-3
Ding, C., Zhu, L., Shen, H., Lu, J., Zou, Q., et al. (2020). Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells, 38(9), 1137–1148. https://doi.org/10.1002/stem.3204
Ding, C., Qian, C., Hou, S., Lu, J., Zou, Q., et al. (2020). Exosomal miRNA-320a Is Released from hAMSCs and Regulates SIRT4 to Prevent Reactive Oxygen Species Generation in POI. Molecular Therapy Nucleic Acids, 21, 37–50. https://doi.org/10.1016/j.omtn.2020.05.013
Thabet, E., Yusuf, A., Abdelmonsif, D. A., Nabil, I., Mourad, G., et al. (2020). Extracellular vesicles miRNA-21: A potential therapeutic tool in premature ovarian dysfunction. Molecular Human Reproduction, 26(12), 906–919. https://doi.org/10.1093/molehr/gaaa068
Huang, B., Lu, J., Ding, C., Zou, Q., Wang, W., et al. (2018). Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Research & Therapy, 9(1), 216. https://doi.org/10.1186/s13287-018-0953-7
Liu, C., Yin, H., Jiang, H., Du, X., Wang, C., et al. (2020). Extracellular Vesicles Derived from Mesenchymal Stem Cells Recover Fertility of Premature Ovarian Insufficiency Mice and the Effects on their Offspring. Cell Transplantation, 29, 963689720923575. https://doi.org/10.1177/0963689720923575
Li, Z., Zhang, M., Zheng, J., Tian, Y., Zhang, H., et al. (2021). Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Improve Ovarian Function and Proliferation of Premature Ovarian Insufficiency by Regulating the Hippo Signaling Pathway. Frontier In Endocrinology (Lausanne), 12, 711902. https://doi.org/10.3389/fendo.2021.711902
Gao, T., Cao, Y., Hu, M., & Du, Y. (2022). Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MicroRNA-29a Improves Ovarian Function of Mice with Primary Ovarian Insufficiency by Targeting HMG-Box Transcription Factor/Wnt/β-Catenin Signaling. Disease Markers, 2022, 5045873. https://doi.org/10.1155/2022/5045873
Qu, Q., Liu, L., Cui, Y., Liu, H., Yi, J., et al. (2022). miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Research & Therapy, 13(1), 352. https://doi.org/10.1186/s13287-022-03056-y
Sun, B., Ma, Y., Wang, F., Hu, L., & Sun, Y. (2019). miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Research & Therapy, 10(1), 360. https://doi.org/10.1186/s13287-019-1442-3
Ma, L. Y. L. Y., H. Li T. (2020). Human Placental Mesenchymal Stem Cells Exosomes for the Repair of Rat Premature Ovarian Failure. World Latest Medicine Information(Chinese) 2020, 20(45), 1–2, 5. doi:https://doi.org/10.3969/j.issn.1671-3141.2020.45.001.
Yang, Z., Du, X., Wang, C., Zhang, J., Liu, C., et al. (2019). Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Research & Therapy, 10(1), 250. https://doi.org/10.1186/s13287-019-1327-5
Geng, Z., Chen, H., Zou, G., Yuan, L., Liu, P., et al. (2022). Human Amniotic Fluid Mesenchymal Stem Cell-Derived Exosomes Inhibit Apoptosis in Ovarian Granulosa Cell via miR-369–3p/YAF2/PDCD5/p53 Pathway. Oxidative Medicine and Cellular Longevity, 2022, 3695848. https://doi.org/10.1155/2022/3695848
Xiao, G.-Y., Cheng, C.-C., Chiang, Y.-S., Cheng, W.T.-K., Liu, I. H., et al. (2016). Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Scientific Reports, 6, 23120. https://doi.org/10.1038/srep23120
Shao, X., Qin, J., Wan, C., Cheng, J., Wang, L., et al. (2021). ADSC Exosomes Mediate lncRNA-MIAT Alleviation of Endometrial Fibrosis by Regulating miR-150–5p. Frontiers In Genetic, 12, 679643. https://doi.org/10.3389/fgene.2021.679643
Xin, L., Wei, C., Tong, X., Dai, Y., Huang, D., et al. (2022). In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: A therapy for intrauterine adhesions. Bioactive Materials, 12, 107–119. https://doi.org/10.1016/j.bioactmat.2021.10.025
Zhang, S., Chang, Q., Li, P., Tong, X., Feng, Y., et al. (2021). Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model. Nanoscale, 13(15), 7334–7347. https://doi.org/10.1039/d0nr08942g
Zhu, Q., Tang, S., Zhu, Y., Chen, D., Huang, J., et al. (2022). Exosomes Derived From CTF1-Modified Bone Marrow Stem Cells Promote Endometrial Regeneration and Restore Fertility. Frontiers In Bioengineering And Biotechnology, 10, 868734. https://doi.org/10.3389/fbioe.2022.868734
Saribas, G. S., Ozogul, C., Tiryaki, M., Alpaslan Pinarli, F., & HamdemirKilic, S. (2020). Effects of uterus derived mesenchymal stem cells and their exosomes on asherman’s syndrome. Acta Histochemica, 122(1), 151465. https://doi.org/10.1016/j.acthis.2019.151465
Xiao, B., Zhu, Y., Huang, J., Wang, T., Wang, F., et al. (2019). Exosomal transfer of bone marrow mesenchymal stem cell-derived miR-340 attenuates endometrial fibrosis. Biology Open, 8(5), bio039958. https://doi.org/10.1242/bio.039958
Zhao, S., Qi, W., Zheng, J., Tian, Y., Qi, X., et al. (2020). Exosomes Derived from Adipose Mesenchymal Stem Cells Restore Functional Endometrium in a Rat Model of Intrauterine Adhesions. Reproductive Sciences, 27(6), 1266–1275. https://doi.org/10.1007/s43032-019-00112-6
Yao, Y., Chen, R., Wang, G., Zhang, Y., & Liu, F. (2019). Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Research & Therapy, 10(1), 225. https://doi.org/10.1186/s13287-019-1332-8
Tan, Q., Xia, D., & Ying, X. (2020). miR-29a in Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Fibrosis during Endometrial Repair of Intrauterine Adhesion. International Journal of Stem Cells, 13(3), 414–423. https://doi.org/10.15283/ijsc20049
Xin, L., Lin, X., Zhou, F., Li, C., Wang, X., et al. (2020). A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomaterialia, 113, 252–266. https://doi.org/10.1016/j.actbio.2020.06.029
Lin, J., Wang, Z., Huang, J., Tang, S., Saiding, Q., et al. (2021). Microenvironment-Protected Exosome-Hydrogel for Facilitating Endometrial Regeneration, Fertility Restoration, and Live Birth of Offspring. Small, 17(11), e2007235. https://doi.org/10.1002/smll.202007235
Wang, X. L., F (2018). Bone marrow mesenchymal stem cell-derived Exosome repair damaged rabbit endometrium. Progress in Obstetrics and Gynecology (Chinese), 2018, 27(3), p 70. https://doi.org/10.13283/j.cnki.xdfckjz.2018.03.003
Ebrahim, N., Mostafa, O., El Dosoky, R. E., Ahmed, I. A., Saad, A. S., et al. (2018). Human mesenchymal stem cell-derived extracellular vesicles/estrogen combined therapy safely ameliorates experimentally induced intrauterine adhesions in a female rat model. Stem Cell Research & Therapy, 9(1), 175. https://doi.org/10.1186/s13287-018-0924-z
Visser, J. A., de Jong, F. H., Laven, J. S. E., & Themmen, A. P. N. (2006). Anti-Müllerian hormone: a new marker for ovarian function. Reproduction (Cambridge, England), 131(1), 1–9. https://pubmed.ncbi.nlm.nih.gov/16388003.
Baek, G., Choi, H., Kim, Y., Lee, H.-C., & Choi, C. (2019). Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cells Translational Medicine, 8(9), 880–886. https://doi.org/10.1002/sctm.18-0226
Raghav, P. K., Mann, Z., Ahlawat, S., & Mohanty, S. (2022). Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. European Journal of Pharmacology, 918, 174657. https://doi.org/10.1016/j.ejphar.2021.174657
Yang, W., Zhang, J., Xu, B., He, Y., Liu, W., et al. (2020). HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Molecular Therapy, 28(4), 1200–1213. https://doi.org/10.1016/j.ymthe.2020.02.003
Mebarki, M., Abadie, C., Larghero, J., & Cras, A. (2021). Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Research & Therapy, 12(1), 152. https://doi.org/10.1186/s13287-021-02222-y
Funding
This study was supported by Kuanren Talents Program of The Second Affiliated Hospital of Chongqing Medical University (to F. H.), and Program for Youth Innovation in Future Medicine, Chongqing Medical University (to F. H.).
Author information
Authors and Affiliations
Contributions
Y.-Y. Z. and L.Q. are responsible for the research, acquisition and data analysis. Y.-Y.Z, F. H. and L.-N.H were in charge of the study design, manuscript writing and revision. S.Y., H.-H.J and L.-Y.J. participated in the data research, analysis, and the interpretation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article, there was a typographical error in the title. Full information regarding the correction made can be found in the erratum/correction for this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Li, Q., You, S. et al. Efficacy of Mesenchymal Stem Cell-Derived Extracellular Vesicles in the Animal Model of Female Reproductive Diseases: A Meta-Analysis. Stem Cell Rev and Rep 19, 2299–2310 (2023). https://doi.org/10.1007/s12015-023-10576-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10576-4