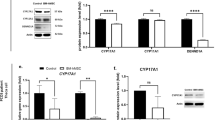
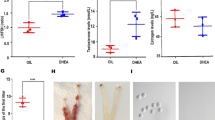
Abstract
Polycystic ovary syndrome (PCOS) is a pathological condition prevalent among women of reproductive age: it is associated with varied etiological factors (lifestyle, genetic, environmental…) and characterized by an increased polycystic morphology of the ovaries leading to disturbances in the menstrual cycle and its correlated infertility. Interconnections between PCOS, obesity, and insulin resistance have been recently investigated thoroughly in the scientific community; these findings directed PCOS therapies into unraveling possibilities to target insulin resistance and central adiposity as efficient treatment. On the other hand, brown adipose tissue is known to possess a thermogenic activity that increases lipolysis and directly attenuates fat deposition. Therefore, brown adipose tissue activation lands itself as a potential target for reducing obesity and its induced insulin resistance, subsequently rescuing PCOS phenotypes. In addition, regenerative medicine has proven efficacy in resolving PCOS-associated infertility and its metabolic symptoms. In particular, many stem/progenitor cells have been verified to possess the differentiation capacity into functional brown adipocytes. Thus, throughout this review, we will discuss the different brown adipose tissue activation strategies and stem-cell-based therapies applied to PCOS models and the possible combination of both therapeutic approaches to synergistically act on the activation of brown adipose tissue and attenuate PCOS-correlated infertility and retract the consequences of the metabolic syndrome on the physiological state of patients.
Graphical Abstract






Similar content being viewed by others
Data Availability
The data used to support the findings of this review are available from the corresponding author upon request.
Code Availability
Not applicable.
Abbreviations
- ACTH :
-
Adrenocorticotropic hormone
- ADSC :
-
Adipose-derived mesenchymal stem cells
- BAT :
-
Brown adipose tissue
- BM-MSC :
-
Bone marrow-derived mesenchymal stem cells
- CVD :
-
Cardiovascular disease
- CYP :
-
Cytochrome P
- DHEA :
-
Dehydroepiandrosterone
- FAH :
-
Functional adrenal hyperandrogenism
- FSH :
-
Follicle stimulating hormone
- GnRH :
-
Gonadotropin-releasing hormone
- iPSC :
-
Induced-pluripotent stem cells
- LH :
-
Luteinizing hormone
- MSC :
-
Mesenchymal stem cells
- PCOM :
-
Polycystic ovary morphology
- PCOS :
-
Polycystic ovary syndrome
- PET :
-
Positron emission tomography
- TC :
-
Thecal cells
- TSC :
-
Thecal stem cells
- UCP-1 :
-
Uncoupling protein-1
References
Deswal, R., Narwal, V., Dang, A., & Pundir, C. S. (2020). The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. Journal of Human Reproductive Sciences, 13(4), 261–271. https://doi.org/10.4103/jhrs.JHRS_95_18
Azziz, R., et al. (2016). Polycystic ovary syndrome. Nature Reviews. Disease Primers, 2(1), 16057. https://doi.org/10.1038/nrdp.2016.57
McCartney, C. R., & Marshall, J. C. (2016). Polycystic Ovary Syndrome. New England Journal of Medicine, 375(1), 54–64. https://doi.org/10.1056/NEJMcp1514916
Ali, A. T. (2015). Polycystic ovary syndrome and metabolic syndrome. Ceska Gynekologie, 80(4), 279–289.
Oliveira, F. R., et al. (2019). Brown adipose tissue in women with polycystic ovary syndrome: Relationship with body measures, plasma irisin levels and the use of metformin. Fertility and Sterility, 112(3), e387–e388. https://doi.org/10.1016/j.fertnstert.2019.07.1108
Shafiee, M. N., Ortori, C. A., Barrett, D. A., Mongan, N. P., Abu, J., & Atiomo, W. (2020). Lipidomic Biomarkers in Polycystic Ovary Syndrome and Endometrial Cancer. International Journal of Molecular Sciences, 21(13), 4753. https://doi.org/10.3390/ijms21134753
Hu, T., et al. (2017). Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. Journal of Nutritional Biochemistry, 47, 21–28. https://doi.org/10.1016/j.jnutbio.2017.04.012
Lu, K.-Y., Dass, K. T. P., Lin, S.-Z., Harn, H.-J., & Liu, S.-P. (2020). The application of stem cell therapy and brown adipose tissue transplantation in metabolic disorders. Cytotherapy, 22(10), 521–528. https://doi.org/10.1016/j.jcyt.2020.06.004
Silva, F., Holt, D., Vargas, V., Bull, D., & Patel, A. N. (2014). Human metabolically active brown adipose tissue derived stem cells. Cytotherapy, 16(4), S69. https://doi.org/10.1016/j.jcyt.2014.01.254
Zhang, Y., Movva, V. C., Williams, M. S., & Lee, M. T. M. (2021). Polycystic Ovary Syndrome Susceptibility Loci Inform Disease Etiological Heterogeneity. Journal of Clinical Medicine, 10(12), 2688. https://doi.org/10.3390/jcm10122688
Merkin, S. S., Phy, J. L., Sites, C. K., & Yang, D. (2016). Environmental determinants of polycystic ovary syndrome. Fertility and Sterility, 106(1), 16–24. https://doi.org/10.1016/j.fertnstert.2016.05.011
Unluturk, U., Harmanci, A., Kocaefe, C., Yildiz, B. O. (2007). The Genetic Basis of the Polycystic Ovary Syndrome: A Literature Review Including Discussion of PPAR-γ. PPAR Research, 2007. https://doi.org/10.1155/2007/49109.
Baskind, N. E., & Balen, A. H. (2016). Hypothalamic–pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Practice & Research. Clinical Obstetrics & Gynaecology, 37, 80–97. https://doi.org/10.1016/j.bpobgyn.2016.03.005
Messinis, I. E. (2006). Ovarian feedback, mechanism of action and possible clinical implications. Human Reproduction Update, 12(5), 557–571. https://doi.org/10.1093/humupd/dml020
Nath, C. K., et al. (2019). Prolactin and thyroid stimulating hormone affecting the pattern of LH/FSH secretion in patients with polycystic ovary syndrome: A hospital-based study from North East India. Journal of Family Medicine and Primary Care, 8(1), 256–260. https://doi.org/10.4103/jfmpc.jfmpc_281_18
Jakimiuk, A. J., Weitsman, S. R., Navab, A., & Magoffin, D. A. (2001). Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. Journal of Clinical Endocrinology and Metabolism, 86(3), 1318–1323. https://doi.org/10.1210/jcem.86.3.7318
Azziz, R., Black, V., Hines, G. A., Fox, L. M., & Boots, L. R. (1998). Adrenal Androgen Excess in the Polycystic Ovary Syndrome: Sensitivity and Responsivity of the Hypothalamic-Pituitary-Adrenal Axis1. Journal of Clinical Endocrinology and Metabolism, 83(7), 2317–2323. https://doi.org/10.1210/jcem.83.7.4948
Chang, R. J., & Cook-Andersen, H. (2013). Disordered follicle development. Molecular and Cellular Endocrinology, 373, 51–60. https://doi.org/10.1016/j.mce.2012.07.011
Tal, R., Seifer, C. M., Khanimov, M., Seifer, D. B., & Tal, O. (2020). High serum Antimullerian hormone levels are associated with lower live birth rates in women with polycystic ovarian syndrome undergoing assisted reproductive technology. Reproductive Biology and Endocrinology, 18(1), 20. https://doi.org/10.1186/s12958-020-00581-4
Goodarzi, M. O., Dumesic, D. A., Chazenbalk, G., & Azziz, R. (2011). Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nature Reviews. Endocrinology, 7(4), 219–231. https://doi.org/10.1038/nrendo.2010.217
Williams, T., Mortada, R., & Porter, S. (2016). Diagnosis and Treatment of Polycystic Ovary Syndrome. American Family Physician, 94(2), 106–113.
Franik, G., et al. (2017). The effect of abdominal obesity in patients with polycystic ovary syndrome on metabolic parameters. European Review for Medical and Pharmacological Sciences, 21(21), 4755–4761.
Tabassum, R., Imtiaz, F., Sharafat, S., Shukar-ud-din, S., & Nusrat, U. (2013). Prevalence and clinical profile of insulin resistance in young women of poly cystic ovary syndrome: A study from Pakistan. Pakistan Journal of Medical Sciences, 29(2), 593–596.
Moghetti, P., & Tosi, F. (2021). Insulin resistance and PCOS: Chicken or egg? Journal of Endocrinological Investigation, 44(2), 233–244. https://doi.org/10.1007/s40618-020-01351-0
Dunaif, A. (1997). Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocrine Reviews, 18(6), 774–800. https://doi.org/10.1210/edrv.18.6.0318
Diamanti-Kandarakis, E., & Dunaif, A. (2012). Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocrine Reviews, 33(6), 981–1030. https://doi.org/10.1210/er.2011-1034
Abel, E. D., et al. (2001). Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature, 409(6821), 729–733. https://doi.org/10.1038/35055575
Bairagi, S., Gopal, J., Nathan, A. A., Babu, S. S., Kumar, N. P., & Dixit, M. (2012). Glucose-induced increase in circulating progenitor cells is blunted in polycystic amenorrhoeic subjects. Human Reproduction, 27(3), 844–853. https://doi.org/10.1093/humrep/der457
Kim, T.-H., et al. (2018). 3D-cultured human placenta-derived mesenchymal stem cell spheroids enhance ovary function by inducing folliculogenesis. Science and Reports, 8, 15313. https://doi.org/10.1038/s41598-018-33575-9
Delitala, A. P., Capobianco, G., Delitala, G., Cherchi, P. L., & Dessole, S. (2017). Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Archives of Gynecology and Obstetrics, 296(3), 405–419. https://doi.org/10.1007/s00404-017-4429-2
Saito, M., Matsushita, M., Yoneshiro, T., Okamatsu-Ogura,Y. (2020). Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Frontiers in Endocrinology, 11. Accessed: Jun. 05, 2022. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fendo.2020.00222.
Saito, M. (2013). Brown Adipose Tissue as a Regulator of Energy Expenditure and Body Fat in Humans. Diabetes and Metabolism Journal, 37(1), 22–29. https://doi.org/10.4093/dmj.2013.37.1.22
Sacks, H., & Symonds, M. E. (2013). Anatomical Locations of Human Brown Adipose Tissue. Diabetes, 62(6), 1783–1790. https://doi.org/10.2337/db12-1430
Lu, W.-H. Chang, Y.-M., Huang, Y.-S. (2021). Alternative Polyadenylation and Differential Regulation of Ucp1: Implications for Brown Adipose Tissue Thermogenesis Across Species. Frontiers in Pediatrics, 8. Accessed: Jun. 05, 2022. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fped.2020.612279.
Cassard, A. M., et al. (1990). Human uncoupling protein gene: Structure, comparison with rat gene, and assignment to the long arm of chromosome 4. Journal of Cellular Biochemistry, 43(3), 255–264. https://doi.org/10.1002/jcb.240430306
Cannon, B., & Nedergaard, J. (2004). Brown adipose tissue: Function and physiological significance. Physiological Reviews, 84(1), 277–359. https://doi.org/10.1152/physrev.00015.2003
Cypess, A. M., & Kahn, C. R. (2010). Brown fat as a therapy for obesity and diabetes. Current Opinion in Endocrinology, Diabetes, and Obesity, 17(2), 143–149. https://doi.org/10.1097/MED.0b013e328337a81f
Li, L., et al. (2020). Characterization of brown adipose tissue (BAT) in polycystic ovary syndrome (PCOS) patients by Z-Spectral Imaging (ZSI). European Journal of Radiology, 123, 108777. https://doi.org/10.1016/j.ejrad.2019.108777
Flávia R, O., et al. (2019). Brown adipose tissue activity is reduced in women with polycystic ovary syndrome. European Journal of Endocrinology, 181(5), 473–480. https://doi.org/10.1530/EJE-19-0505.
Patel, K., Patel, D. K. (2019). Chapter 26 - The Beneficial Role of Rutin, A Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In: Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases (Second Edition), R. R. Watson and V. R. Preedy, Eds. Academic Press, pp. 457–479. https://doi.org/10.1016/B978-0-12-813820-5.00026-X.
Yuan, X., et al. (2017). Rutin ameliorates obesity through brown fat activation. FASEB Journal: official publication of the Federation of American Societies for Experimental Biology, 31(1), 333–345. https://doi.org/10.1096/fj.201600459RR
Sharifi-Rad, J., et al. (2020). Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Frontiers in Pharmacology, 11, Accessed: Jun. 05, 2022. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fphar.2020.01021.
Wang, S., et al. (2015). Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochemical and Biophysical Research Communications, 466(2), 247–253. https://doi.org/10.1016/j.bbrc.2015.09.018
Kamatou, G. P. P., Vermaak, I., Viljoen, A. M., & Lawrence, B. M. (2013). Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry, 96, 15–25. https://doi.org/10.1016/j.phytochem.2013.08.005
Ma, S., et al. (2012). Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. Journal of Molecular Cell Biology, 4(2), 88–96. https://doi.org/10.1093/jmcb/mjs001
Wang, S., et al. (2015). Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. International Journal of Obesity, 39(6), 6. https://doi.org/10.1038/ijo.2015.23.
Dulloo, A. G., et al. (1999). Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. American Journal of Clinical Nutrition, 70(6), 1040–1045. https://doi.org/10.1093/ajcn/70.6.1040
Kawabata, F., et al. (2009). Non-Pungent Capsaicin Analogs (Capsinoids) Increase Metabolic Rate and Enhance Thermogenesis via Gastrointestinal TRPV1 in Mice. Bioscience, Biotechnology, and Biochemistry, 73(12), 2690–2697. https://doi.org/10.1271/bbb.90555
Yao, L., et al. (2021). Brown Adipose Transplantation Improves Polycystic Ovary Syndrome-Involved Metabolome Remodeling. Frontiers in Endocrinology, 12, 747944. https://doi.org/10.3389/fendo.2021.747944
Du, L., et al. (2021). Rat BAT xenotransplantation recovers the fertility and metabolic health of PCOS mice. Journal of Endocrinology, 248(2), 249–264. https://doi.org/10.1530/JOE-20-0068
Yuan, X., et al. (2016). Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proceedings of the National Academy of Sciences, 113(10), 2708–2713. https://doi.org/10.1073/pnas.1523236113
Zhang, Q., et al. (2022) Brown Adipose Tissue and Novel Management Strategies for Polycystic Ovary Syndrome Therapy. Frontiers in Endocrinology, 13. Accessed: Jun. 06, 2022. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fendo.2022.847249.
Ye, R., et al. (2021) Brown Adipose Tissue Activation by Cold Treatment Ameliorates Polycystic Ovary Syndrome in Rat. Frontiers in Endocrinology, 12. https://doi.org/10.3389/fendo.2021.744628.
Ee, C. et al. (2018). Feasibility and acceptability of a proposed trial of acupuncture as an adjunct to lifestyle interventions for weight loss in Polycystic Ovary Syndrome: a qualitative study. BMC Complementary and Alternative Medicine, 18. https://doi.org/10.1186/s12906-018-2358-7.
Gao, H., et al. (2022). Three-dimensional visualization of electroacupuncture-induced activation of brown adipose tissue via sympathetic innervation in PCOS rats. Chinese Medicine, 17(1), 48. https://doi.org/10.1186/s13020-022-00603-w
Piltonen, T. T., et al. (2013). Mesenchymal Stem/Progenitors and Other Endometrial Cell Types From Women With Polycystic Ovary Syndrome (PCOS) Display Inflammatory and Oncogenic Potential. Journal of Clinical Endocrinology and Metabolism, 98(9), 3765–3775. https://doi.org/10.1210/jc.2013-1923
Min, Z., et al. (2018). New insights into the genic and metabolic characteristics of induced pluripotent stem cells from polycystic ovary syndrome women. Stem Cell Research & Therapy, 9(1), 210. https://doi.org/10.1186/s13287-018-0950-x
Sánchez, A., Schimmang, T., & García-Sancho, J. (2012). Cell and tissue therapy in regenerative medicine. Advances in Experimental Medicine and Biology, 741, 89–102. https://doi.org/10.1007/978-1-4614-2098-9_7
Mao, A. S., & Mooney, D. J. (2015). Regenerative medicine: Current therapies and future directions. Proceedings of the National Academy of Sciences of the United States of America, 112(47), 14452–14459. https://doi.org/10.1073/pnas.1508520112
Park, H.-S., et al. (2022). Suppression pathway of androgen production through secreted proteins in msc based pcos treatment. Fertility and Sterility, 118(4), Supplement, e347. https://doi.org/10.1016/j.fertnstert.2022.09.148.
Kalhori, Z., Azadbakht, M., Soleimani Mehranjani, M., Shariatzadeh, M. A. (2018) Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy, 20(12), 1445–1458. https://doi.org/10.1016/j.jcyt.2018.09.005.
Chugh, R. M., et al. (2021). Mesenchymal stem cell therapy ameliorates metabolic dysfunction and restores fertility in a PCOS mouse model through interleukin-10. Stem Cell Research & Therapy, 12, 388. https://doi.org/10.1186/s13287-021-02472-w
Chugh, R. M., Park, H., Esfandyari, S., Elsharoud, A., Ulin, M., & Al-Hendy, A. (2021). Mesenchymal Stem Cell-Conditioned Media Regulate Steroidogenesis and Inhibit Androgen Secretion in a PCOS Cell Model via BMP-2. International Journal of Molecular Sciences, 22(17), 9184. https://doi.org/10.3390/ijms22179184
Park, H.-S., et al. (2022). Non-Cytokine Protein Profile of the Mesenchymal Stem Cell Secretome That Regulates the Androgen Production Pathway. International Journal of Molecular Sciences, 23(9), 4633. https://doi.org/10.3390/ijms23094633
Xie, Q., et al. (2019). Mesenchymal Stem Cells Alleviate DHEA-Induced Polycystic Ovary Syndrome (PCOS) by Inhibiting Inflammation in Mice. Stem Cells International, 2019, e9782373. https://doi.org/10.1155/2019/9782373
Julania, S., Walls, M. L., & Hart, R. (2018). The Place of In Vitro Maturation in PCO/PCOS. International Journal of Endocrinology, 2018, 5750298. https://doi.org/10.1155/2018/5750298
Jafarzadeh, H., Nazarian, H., Ghaffari Novin, M., Shams Mofarahe, Z., Eini, F., Piryaei, A. (2018) Improvement of oocyte in vitro maturation from mice with polycystic ovary syndrome by human mesenchymal stromal cell–conditioned media. Journal of Cellular Biochemistry, 119(12), 10365–10375. https://doi.org/10.1002/jcb.27380.
Zhao, Y., Tao, M., Wei, M., Du, S., Wang, H., & Wang, X. (2019). Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artificial Cells, Nanomedicine, and Biotechnology, 47(1), 3804–3813. https://doi.org/10.1080/21691401.2019.1669619
Cao, M., et al. (2022). Adipose mesenchymal stem cell–derived exosomal microRNAs ameliorate polycystic ovary syndrome by protecting against metabolic disturbances. Biomaterials, 288, 121739. https://doi.org/10.1016/j.biomaterials.2022.121739
Zhao, Y., Pan, S., & Wu, X. (2022). Human umbilical cord mesenchymal stem cell-derived exosomes inhibit ovarian granulosa cells inflammatory response through inhibition of NF-κB signaling in polycystic ovary syndrome. Journal of Reproductive Immunology, 152, 103638. https://doi.org/10.1016/j.jri.2022.103638
Park, H., Ashour, D., Elsharoud, A., Chugh, R., Al-Hendy, A., El Andaloussi, A. (2019) Towards stem cell therapy of polycystic ovary syndrome (PCOS): therapeutic effect of human mesenchymal stem cells transplantation in pcos mouse model by regulating ovarian vascularization. Cytotherapy, 21(5, Supplement), S78. https://doi.org/10.1016/j.jcyt.2019.03.484.
Li, Y., Guo, J., Deng, S., Gao, Z., Liu, Y., Gu, Q. (2020) Fibrin Facilitates Mesenchymal Stem Cells to Ameliorate Rats with Polycystic Ovary Syndrome. Applied Sciences, 10(10), 10. https://doi.org/10.3390/app10103598.
Chen, H., et al. (2021). Autologous transplantation of thecal stem cells restores ovarian function in nonhuman primates. Cell Discovery, 7(1), 75. https://doi.org/10.1038/s41421-021-00291-0
Wang, C.-H., et al. (2020). CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Science Translational Medicine, 12(558), eaaz8664. https://doi.org/10.1126/scitranslmed.aaz8664.
Carobbio, S., Rosen, B., & Vidal-Puig, A. (2013). Adipogenesis: New insights into brown adipose tissue differentiation. Journal of Molecular Endocrinology, 51(3), T75–T85. https://doi.org/10.1530/JME-13-0158
Calvo, E., et al. (2021) Effects of stem cells from inducible brown adipose tissue on diet-induced obesity in mice. Scientific Report, 11(1), 1. https://doi.org/10.1038/s41598-021-93224-6.
Zhao, H., et al. (2018). Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes, 67(2), 235–247. https://doi.org/10.2337/db17-0356
Roman, S., et al. (2015). Brown adipose tissue and novel therapeutic approaches to treat metabolic disorders. Translational Research, 165(4), 464–479. https://doi.org/10.1016/j.trsl.2014.11.002
Acknowledgements
We would like to thanks Edde Associtation – Lebanon for their support to LIT Laboratory. We would like to thank Dr Rim Nassar for the technical support she gave concerning the Biorender logiciel. Also, we would like to thank the Lebanese University for all the support. The English grammar and spelling of this manuscript have been professionally edited by Mrs Ferial Srour, we would like to thank her for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
Fadia Najjar and Aline Hamade had the idea for the article. All the authors performed the literature search and data analysis. The first draft of the manuscript was written by Mario Karam. Hélène Najjar, Marwan El Sabban, Aline Hamade and Fadia Najjar critically revised the manuscript for intellectual content. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Conflicts of Interests/Competing Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karam, M., Najjar, H., El Sabban, M. et al. Regenerative Medicine for Polycystic Ovary Syndrome: Stem Cell-Based Therapies and Brown Adipose Tissue Activation. Stem Cell Rev and Rep 19, 853–865 (2023). https://doi.org/10.1007/s12015-023-10505-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10505-5